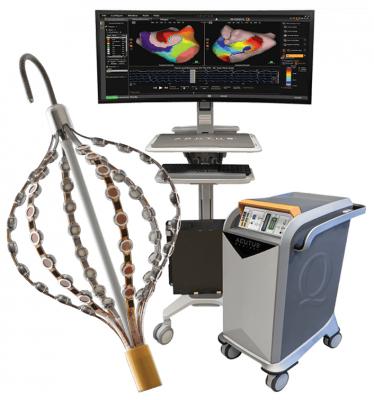
September 29, 2020 — The U.S. Food and Drug Administration (FDA) granted 510(k) clearance for the second-generation of the Acutus Medical AcQMap 3D imaging and mapping catheter. The new version of the electrophysiology (EP) mapping system has improving handling and deliverability.
This flagship 3-D mapping and navigation catheter combines 48 ultrasound transducers responsible for creating the anatomical geometry and 48 engineered electrodes, which enable electrical activation patterns to be displayed along the inner surface of the heart. Compatible with an 0.035” guidewire, the AcQMap catheter is designed to be inserted into the left and right atrium.
The AcQMap catheter is the cornerstone of Acutus’ innovative mapping platform. It is the world’s only integrated high-resolution ultrasound-based imaging and non-contact mapping catheter capable of capturing cardiac imaging information in addition to cardiac activation mapping. This second-generation catheter carries additional benefits such as improved anatomy reconstruction, faster acquisition times and the potential for enhanced procedural efficiencies. The catheter also comes with a reusable and sterilizable cable that can be used up to 10 times, resulting in greater cost savings for health care systems.
Customer feedback during development suggested that users perceived anatomy reconstruction to be faster than our generation one catheter.
“The new ergonomic handle and catheter deployment mechanism has the potential to allow me to maneuver the catheter with greater speed, ease and control,” explained Anil Rajendra, M.D., FACC, and electrophysiologist from Grandview Medical Center in Birmingham, Alabama.
The versatility of the AcQMap System allows physicians to map all types of atrial arrhythmias – simple and complex, spontaneous, repetitive and chaotic. The high definition maps provided by the AcQMap catheter allow physicians to identify ablation targets outside the pulmonary veins and strategically plan a patient-specific ablation strategy. Physicians can efficiently map any arrhythmia in under 3 minutes, ablate and then remap again to evaluate therapy effectiveness.
For more information: www.acutusmedical.com/us


 December 19, 2025
December 19, 2025 









