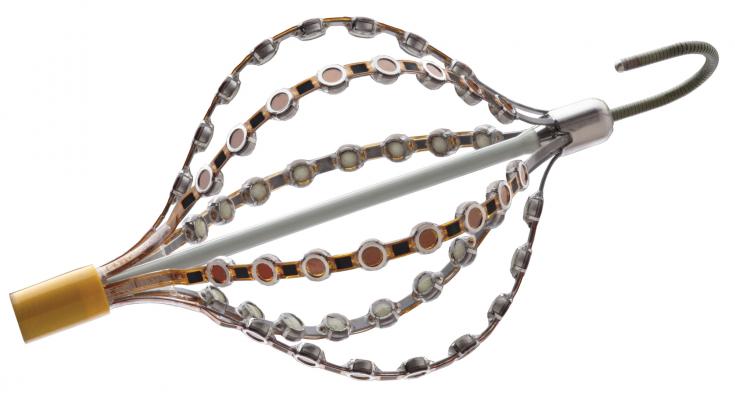
October 9, 2019 — Acutus Medical and Innovative Health announced a partnership that will offer advanced electrophysiology technology to improve patient outcomes in a cost-efficient manner. The two high profile venture-backed companies will team to offer hospital customers a comprehensive suite of EP products, including Acutus’ next-generation EP mapping and accessory products together with a growing portfolio of reprocessed single-use EP devices from a range of manufacturers.
Acutus will also work proactively with Innovative Health to gain clearances, as appropriate, to market reprocessed versions of certain proprietary Acutus products – a highly novel approach for a venture-backed device company. The goal of the partnership is to simultaneously rein in per-procedure costs while freeing up hospital budget dollars for emerging new technologies that hold the prospect of improving clinical outcomes and speeding up procedure times.
Recognizing the constraints that hospitals and health systems face in delivering quality patient care at a sustainable cost is fundamental in the current healthcare environment. Safe, proven and effective reprocessing of high-cost single-use devices offers a reliable avenue for hospitals to combine the best of both worlds: best-in-class, state-of-the-art technologies together with cost-effective, sustainable per-procedure costs.
Acutus provides a full suite of technologies and products for electrophysiology diagnostic and ablation procedures through an open platform that enables personalized and adaptive approaches to arrhythmia therapy.
Innovative Health is a specialty EP reprocessing company that collects, reprocesses and returns single-use labeled EP catheters to hospitals, reducing each atrial fibrillation (AF) procedure’s device costs by as much as 30 percent. The company holds formal U.S. Food and Drug Administration (FDA) clearances to reprocess many high-complexity EP devices, such as mapping catheters, diagnostic ultrasound catheters and introducer sheaths. This allows Innovative Health hospital partners to increase their savings compared to other reprocessing programs.
For more information: www.acutusmedical.com, www.innovative-health.com


 January 29, 2026
January 29, 2026 









