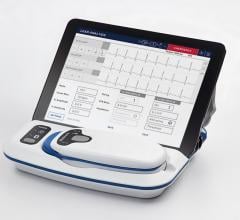
February 2, 2024 — GE HealthCare (Nasdaq: GEHC) announces the latest innovation in electrophysiology (EP), the Prucka 3 with CardioLab EP Recording system, to help clinicians in the diagnosis and treatment of cardiac arrhythmias.
Cardiovascular disease is the leading cause of death globally - affecting millions of individuals and placing a significant burden on healthcare systems today.[1] Within the complex umbrella of cardiology care and cardiovascular disease, atrial fibrillation (AFib) is the most common arrhythmia diagnosed in clinical practice with projections indicating that the prevalence of AFib could reach 15.9 million people in the United States by 2050 and 17.9 million in Europe by 2060.[2] For those living with cardiac arrhythmias like AFib, the longer a person is living out of rhythm, the more difficult the arrhythmia is to treat and the more dangerous it may become. If diagnosed early, rhythm control treatment can reduce AFib recurrence and potentially prevent progression.[3]
Electrophysiology labs play a pivotal role in the treatment of cardiac arrhythmias like AFib and require sophisticated recording systems to capture and analyze intracardiac signals accurately. GE HealthCare’s latest Prucka 3 with CardioLab EP Recording system builds on more than twenty years of success in the field of electrophysiology to provide a robust ecosystem of mapping technologies that help provide accurate, efficient, and advanced analytics for the diagnosis and treatment of cardiac arrhythmias.
The system’s digital platform maintains high signal fidelity, while reducing environmental noise through new signal-filtering capabilities and enhanced software for powerline noise reduction.[4] The forward-looking platform also provides a path to emerging technologies in electrophysiology that depend on quality signals for their fast interpretation and clinical results.
“Signal clarity is key in the EP Lab,” shares Dr. Usman Siddiqui, Director of Electrophysiology.[5] “With the launch of the latest Prucka 3 digital amplifier, I feel confident analyzing critical signals regardless of the type of ablation technology or platform I am using. Prucka 3 delivers the signal quality I expect from CardioLab and GE HealthCare.”
‘‘The launch of the latest Prucka 3 digital amplifier is a game changer for EP labs,” shares Devon Bream, General Manager of Invasive Cardiology for GE HealthCare. “The new platform not only provides beautiful signals, but the digital architecture continues to demonstrate our commitment to give clinicians the tools, along with the flexibility and adaptability, to enable better outcomes for their patients. After serving the EP community for over 20 years, the latest Prucka 3 with CardioLab truly ‘amplifies’ the EP Lab experience through a fully digital experience ready to enable the future of electrophysiology.”
New features now available as part of the latest Prucka 3 with CardioLab EP Recording system:
- Enhanced Signal Filtering Capabilities including powerline harmonic removal;
- Dual Real-Time places a second real-time display on any of the CardioLab display monitors;
- Cycle Length on Signal labels the cycle length directly on the signal in real-time;
- Multiple Review Windows allow for the placement of up to five review windows; and
- ClearMatch tags interesting morphology to create a template that can be used in review to match the two morphologies.
Prucka 3 with CardioLab EP Recording system is part of GE HealthCare’s larger cardiology care portfolio to address cardiovascular disease and key challenges faced in cardiology care today. As part of this comprehensive care pathway approach, GE HealthCare has made other recent enhancements to the portfolio including the introduction of Allia IGS Pulse, the next generation of image-guided systems designed for cardiac imaging excellence and CardioVisio for Atrial Fibrillation which helps visualize the history of the patient’s heart and provides guideline-directed insights to healthcare providers.
For more information: www.geheathcare.com
References:
[1] World Health Organization (WHO) estimated 17.9 million people died from cardiovascular diseases in 2019. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
[2] Vinter N, Huang Q, Fenger-Grøn M, Frost L, Benjamin EJ, Trinquart L. Trends in excess mortality associated with atrial fibrillation over 45 years (Framingham Heart Study): community based cohort study. BMJ. 2020 Aug 11;370:m2724. doi: 10.1136/bmj.m2724. PMID: 32784208; PMCID: PMC7418071.
[3] Camm A, Naccarelli G, Mittal S, et al. The Increasing Role of Rhythm Control in Patients With Atrial Fibrillation. J Am Coll Cardiol. 2022 May, 79 (19) 1932–1948. https://doi.org/10.1016/j.jacc.2022.03.337.
[4] GE HealthCare Data on File
[5] Dr. Siddiqui is a paid consultant for GE HealthCare and was compensated for participation in this testimonial. The statements by Dr. Siddiqui described here are based on his own opinions and on results that were achieved in his unique setting. Since there is no “typical” hospital and many variables exist, (i.e., hospital size, case mix, etc.) there can be no guarantee that other customers will achieve the same results.
[6] Dr. Oza is a paid consultant for GE HealthCare and was compensated for participation in this testimonial. The statements by Dr. Oza described here are based on his own opinions and on results that were achieved in his unique setting. Since there is no “typical” hospital and many variables exist, (i.e., hospital size, case mix, etc.) there can be no guarantee that other customers will achieve the same results.


 May 03, 2023
May 03, 2023