Every second counts when someone is having a stroke and immediate care can mean the difference between life and death. While there is widespread consensus on the importance of identification and ...
Implantable Cardiac Monitor (ICM)
Implantable cardiac monitors (ICM) are small electrophysiology (EP) devices that used for long-term monitoring of a patient's heart electrical activity to detect arrhythmias. The technology can eliminate the need for a bulkly external Holter monitor that requires wire leads attached to the patient. ICMs can be inserted under the skin during and office visit and record cardiac data continuously for up to 4.5 years.
Every second counts when someone is having a stroke and immediate care can mean the difference between life and death ...
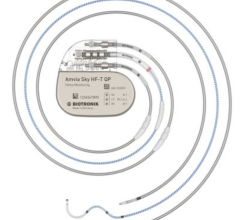
July 11, 2024 — Dr. Fadi Mansour performed the first Canadian implant of BIOTRONIK’s newest pacemaker and CRT-P ...
February 15, 2024 — Canary Medical, a medical data company focused on the development and commercialization of its ...
January 15, 2024 — A public-private partnership Think Tank, scheduled for Feb. 29-March 1, will be presented by the Card ...
This past spring, electrophysiology experts issued new recommendations for remote monitoring of cardiovascular ...
October 3, 2023 -- Boston Scientific launched the LUX-Dx II+ Insertable Cardiac Monitor (ICM) System, a next-generation ...

The global cardiovascular diagnostic and monitoring devices market was valued at $14.2B in 2021 and is projected to ...
June 1, 2023 — Every year more than one million people receive a pacemaker. Until now, leadless versions were only ...
May 18, 2023 — Abbott announced its Assert-IQ insertable cardiac monitor (ICM) has received U.S. Food and Drug ...

The global ambulatory diagnostic cardiology market was valued at $2.6 billion in 2022 and is forecast to rise to $3.3 ...
May 4, 2023 — Avertix Medical, Inc., formerly known as Angel Medical Systems, Inc., a company focused on improving long ...
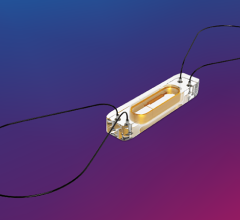
April 20, 2023 — Researchers led by Northwestern University and the University of Texas at Austin (UT) have developed ...
March 24, 2023 — Abbott announced new data that found monitoring patients remotely with hemodynamic pressure sensing ...
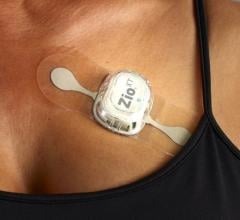
March 3, 2023 — iRhythm Technologies, Inc. , a leading digital health company focused on creating trusted solutions ...
February 22, 2023 — CVRx, Inc., a commercial-stage medical device company focused on developing, manufacturing and ...



 July 02, 2025
July 02, 2025