February 10, 2023 — Irregular heart rhythms were detected in about 1 in 5 people who survived an ischemic stroke due to ...
Implantable Cardiac Monitor (ICM)
Implantable cardiac monitors (ICM) are small electrophysiology (EP) devices that used for long-term monitoring of a patient's heart electrical activity to detect arrhythmias. The technology can eliminate the need for a bulkly external Holter monitor that requires wire leads attached to the patient. ICMs can be inserted under the skin during and office visit and record cardiac data continuously for up to 4.5 years.
January 30, 2023 — Heart disease is the leading cause of death worldwide. In the U.S., it is estimated that someone dies ...
October 6, 2022 — The complications experienced by heart failure patients implanted with a left ventricular assist ...
September 20, 2022 — Medtronic, a global leader in healthcare technology, today announced the LINQ II Insertable Cardiac ...

The current standard of care for heart failure (HF) is guideline-directed medical therapy combined with device-based ...
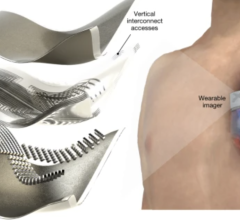
May 4, 2022 — More than 60 million magnetic resonance imaging (MRI) scans are performed worldwide each year, but imaging ...
April 29, 2022 – Medtronic has announced new data from the STROKE AF clinical trial, which showed that large and small ...
April 6, 2022 – Final results from the BIO|GUARD-MI study show a 31 percent reduction of MACE in a sub-group analysis of ...
February 25, 2022 – Abbott has announced that the U.S. Food and Drug Administration (FDA) has approved an expanded ...
January 4, 2022 – Remote patient monitoring and cardiac data management solutions vendor Implicity received U.S. Food ...
September 8, 2021 — Continuous heart rhythm monitoring – with anticoagulation if atrial fibrillation is detected – does ...
September 7, 2021 — Remote monitoring of implantable cardiac monitors (ICMs) is highly effective for early detection of ...
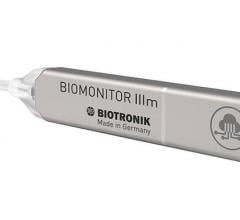
August 9, 2021 - In association with Heart Rhythm 2021, Biotronik today announced that the latest implantable cardiac ...

Cardiovascular diseases are among the leading causes of death for people over 65 years old in North America and Europe ...
June 13, 2021 — Medtronic announced clinical trial results from the STROKE AF trial demonstrating the superiority of the ...

 February 10, 2023
February 10, 2023