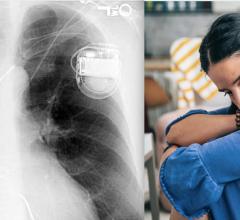
July 1, 2021 — The U.S. Food and Drug Administration (FDA) has cleared the Angel Medical Systems Inc. second-generation ...
Implantable Cardiac Monitor (ICM)
Implantable cardiac monitors (ICM) are small electrophysiology (EP) devices that used for long-term monitoring of a patient's heart electrical activity to detect arrhythmias. The technology can eliminate the need for a bulkly external Holter monitor that requires wire leads attached to the patient. ICMs can be inserted under the skin during and office visit and record cardiac data continuously for up to 4.5 years.
June 2, 2021 — Implicity, a leader in remote patient monitoring software and cardiac data management solutions ...
May 14, 2021 — The U.S. Food and Drug Administration (FDA) is advising patients and caregivers to keep any consumer ...
April 26, 2021 — Patients receiving an implantable cardioverter defibrillator (ICD) should be regularly screened for ...

A few weeks ago, a patient of mine experienced a cardiac event — his heart stopped. Completely. However, his implantable ...
February 2, 2021 – Optimize EP, a digital health company focused on transforming cardiac care by improving the current ...
December 22, 2020 — The U.S. Food and Drug Administration (FDA) cleared Biotronik's Vital Data Sensor, which identifies ...
Robert Kowal, M.D., chief medical officer of the Medtronic cardiac rhythm and heart failure division, said there has ...
July 7, 2020 – Medtronic announced it received U.S. Food and Drug Administration (FDA) clearance and European CE mark ...
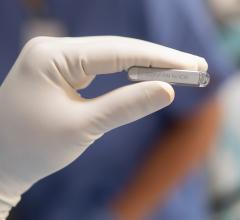
June 29, 2020 — Boston Scientific received U.S. Food and Drug Administration (FDA) 510(k) clearance for the LUX-Dx Inser ...
The Consumer Electronic Show (CES) is the world's gathering place for consumer technologies, with more than 175,000 ...
July 9, 2019 — South Australian researchers are developing a tiny fiber-optic sensor, which has the potential to change ...
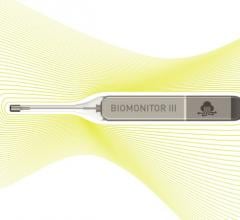
July 1, 2019 – Biotronik announced the European market release of its new injectable cardiac monitor (ICM), Biomonitor ...
May 6, 2019 — Abbott announced the launch of a new, smarter heart monitor for better arrhythmia detection — positive ...
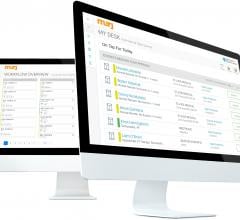
May 1, 2019 — Digital healthcare company Murj announced the immediate availability of the Murj 2.0 implantable cardiac ...


 July 01, 2021
July 01, 2021