
Image courtesy of Flinders University
July 9, 2019 — South Australian researchers are developing a tiny fiber-optic sensor, which has the potential to change the way blood flow is monitored. The device is so small that it can potentially be used to monitor newborn and premature babies.
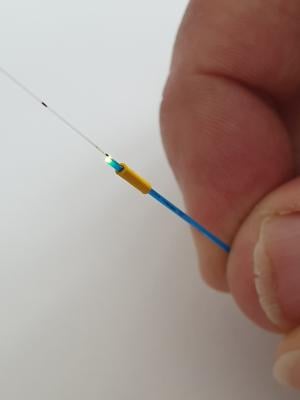
Researchers from Flinders University in Adelaide have filed a provision patent for the micro-medical device that can continuously measure blood flow through the aorta and in real-time.
Research leader Strategic Professor John Arkwright said the device could be a game-changer for people in intensive care and undergoing surgical procedures.
“It’s a far more responsive measurement compared to traditional blood flow monitoring – and without life-threatening delays in the period snapshot provided by current blood flow practices using ultrasound or thermo-dilution,” he said.
But Flinders University biomedical engineer Albert Ruiz-Vargas, Ph.D., who helped develop the device, said additional funding was needed to engineer the device.
The project’s chief investigator said more research was required to determine how the sensor would behave under physiological conditions and to examine different encapsulations.
“The proof-of-concept prototype is potentially a low-cost device which has passed initial testing in a heart-lung machine,” Ruiz-Vargas said. “It can be inserted through a small keyhole aperture in the skin into the femoral artery in individuals where heart function is compromised and is so small it can even measure small changes in flow in the tiny blood vessels of infants.
“It’s a simple design, which can give readouts similar to a pulsating heartbeat response on a laptop or nearby screen.”
While the cardiac flow monitoring probe was developed for use in neonates in the intensive care unit, it could be used for patients of all ages, including cardiac bypass patients.
“Basically, neonates are prone to having more changes in blood flow and that can mean damage to any organs or the brain, because their vessels are more fragile,” Ruiz-Vargas said. “What we’ve got so far, it gives us a measurement every 30 minutes and has a lot of operator errors. With our device that won’t happen because it doesn’t need expertise (to control it). You just put it inside the body and it will measure continuously and all you need to do is watch the blood flow.”
He said he hoped the device would be picked up for further development and introduction into regular intensive care and surgical procedures.
“Optical flow sensor for continuous invasive measurement of blood flow velocity” was published in the Journal of Biophotonics.
For more information: www.onlinelibrary.wiley.com
Reference
1. Ruiz-Vargas A., Morris S.A., Hartley R.H., et al. Optical flow sensor for continuous invasive measurement of blood flow velocity. Journal of Biophotonics, May 18, 2019. https://doi.org/10.1002/jbio.201900139


 November 12, 2025
November 12, 2025 









