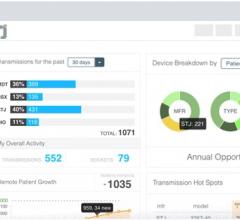
Innovation in medical technology exists to improve patient outcomes, increase surgical efficiency and enhance patient safety. Infrequently, we see medical technology innovation of a paradigmatic ...
EP Device Monitoring Systems
EP device monitoring systems allow for cardiac electrophysiology devices to be monitored.
Aug. 18, 2025 — iRhythm Technologies, Inc. has announced the publication of a real-world evidence study: Assessment of V ...

Innovation in medical technology exists to improve patient outcomes, increase surgical efficiency and enhance patient ...
February 2, 2024 — GE HealthCare (Nasdaq: GEHC) announces the latest innovation in electrophysiology (EP), the Prucka 3 ...
May 3, 2023 — BioSig Technologies, Inc., a medical technology company delivering unprecedented accuracy and precision to ...
May 2, 2023 — The Heart Rhythm Society (HRS) is making final preparations for its Annual Heart Rhythm Meeting, HRS2023 ...
January 13, 2023 — BioSig Technologies, Inc., an advanced digital signal processing technology company delivering ...
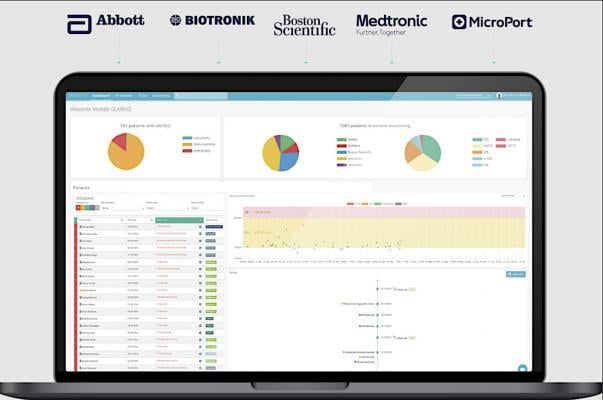
With 1.2 to 1.4 million new electrophysiology (EP) devices being prescribed to patients around the world each year ...

Cardiovascular diseases are among the leading causes of death for people over 65 years old in North America and Europe ...
June 2, 2021 — Implicity, a leader in remote patient monitoring software and cardiac data management solutions ...

A few weeks ago, a patient of mine experienced a cardiac event — his heart stopped. Completely. However, his implantable ...
Robert Kowal, M.D., chief medical officer of the Medtronic cardiac rhythm and heart failure division, said there has ...
May 14, 2020 – Medtronic announced results from late-breaking clinical trials evaluating the MyCareLink Heart mobile app ...
October 31, 2019 — AliveCor, a provider of smartphone based ECG technology and Huami Corp., a provider of biometric ...
May 17, 2019 — Digital healthcare company Murj announced the availability of the Murj Analytics software-as-a-service ...
May 2, 2019 — Medtronic announced the company has received U.S. Food and Drug Administration (FDA) approval for the ...





 August 19, 2025
August 19, 2025