Oct. 21, 2025 — GE HealthCare has received CE mark for its Carevance patient monitor, advancing accessible and reliable care for more customers starting in Europe. Carevance offers a clinically ...
Patient Monitors
Oct. 21, 2025 — GE HealthCare has received CE mark for its Carevance patient monitor, advancing accessible and reliable ...
Sept. 25, 2025 — Royal Philips has entered a national partnership in the United States with Optum Healthcare. The ...
Sept. 4, 2025 — Royal Philips has introduced a new telemetry platform designed to help address critical challenges in ...
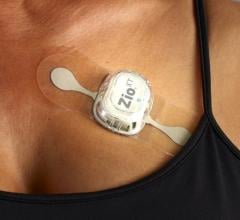
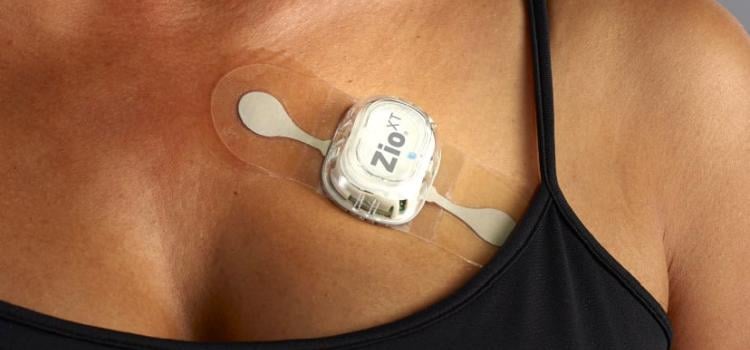
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
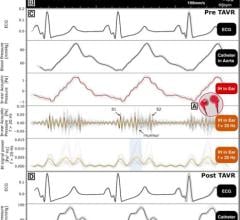
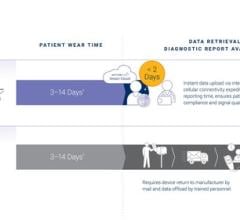
Aug. 18, 2025 — iRhythm Technologies, Inc. has announced the publication of a real-world evidence study: Assessment of V ...
March 5, 2024 — FIRE1 announced that it has completed patient enrollment in the U.S. Early Feasibility Study (FUTURE ...
January 3, 2024 — iRhythm Technologies, Inc., a leading digital health care company focused on creating trusted ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
December 26, 2023 — MindMics, Inc. reported results from a clinical study of revolutionary earbuds that use a new ...
July 11, 2023 — FIRE1 announced that the first U.S. patients have been successfully implanted with its FIRE1 System for ...
July 5, 2023 — Heart health risks emerge early in life in American Indian/Alaska Native (AI/AN) women and are increased ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
May 2, 2023 — Ambiq, a leading developer of ultra-low-power semiconductor solutions that deliver a multifold increase in ...
February 21, 2023 — AliveCor, the global leader in FDA-cleared personal electrocardiogram (ECG) technology, today ...
October 25, 2022 — RhythMedix, a leader in remote cardiac monitoring and proprietary arrhythmia detection algorithms ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The newer ECG devices we have now are so much less cumbersome. It’s like wearing a Band-Aid versus carrying a bulky device,” said the Greenwood, Mississippi internist. “My patients prefer the more comfortable, wire-free form factor, and the quality is as good as, or better, than the Holter,” continued Boler. “Plus, my patient compliance has increased. With the Holter, the leads sometimes come off. The patient may think the device isn’t working, so they take it off and we have to restart the process.”
September 19, 2022 — HeartBeam, Inc. (NASDAQ: BEAT), a cardiac technology company that has developed the first and only ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...




 October 21, 2025
October 21, 2025