December 26, 2023 — MindMics, Inc. reported results from a clinical study of revolutionary earbuds that use a new technology - In-ear Infrasonic Hemodynography to Detects Aortic Stenosis Murmur Before and After Transcatheter Aortic Valve Replacement (TAVR). These results were published by Journal of the American College of Cardiology (JACC): Case Reports Volume 28, Part 1, 20 December 2023, 102089: https://www.sciencedirect.com/science/article/pii/S2666084923004254?via%3Dihub
Results from an ongoing prospective clinical study conducted by Scripps Clinic indicate that MindMics' non-invasive infrasonic hemodynography (IH) technology can detect aortic stenosis by capturing in-ear acoustic vibrations throughout the cardiac cycles.
"Aortic Stenosis (AS) produces a typical systolic ejection sound detected on clinical auscultation," said Dr. Christine Shen MD, one of the study's authors. "We propose a new method of assessing aortic stenosis through IH to detect its characteristic systolic ejection murmur."
Anna Barnacka, PhD, CEO of MindMics, added "Our technology platform offers a convenient and accessible way of building towards a future where cardiac care could be possible through a pair of everyday earbuds. The potential to detect aortic stenosis through our IH technology is an exciting development that brings us a step closer to achieving the MindMics purpose to one day eliminate heart failure as the leading cause of death globally (similar to what glucose monitoring has done to transform diabetes)."
"We are grateful to our colleagues at Scripps and the patient for an opportunity to collect data during a highly invasive procedure of transcatheter aortic valve replacement using our non-invasive in-ear device. We are excited to see that the features observed in our data closely follow those of the arterial pressure recorded with the cardiac catheter, both before and after the procedure of valve replacement. In addition, the data showed that our device is able to detect a characteristic structure of the AS heart murmur present before the procedure. This gives us strong motivation to continue our studies and expand them to include other types of heart murmurs, " said Robert Ciesielski, Ph.D., the R&D Manager of MindMics.
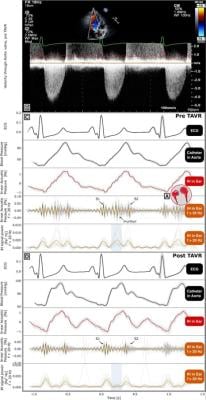
In the study, a patient with severe aortic stenosis undergoing transcatheter aortic valve replacement (TAVR) wore the MindMics IH earbuds and additionally underwent simultaneous measurements with earbuds and echocardiography. The resulting IH waveform was limited to frequencies above 20 Hz.
"Results showed that signals were detected at the start and end of systole, when the first (S1) and the second (S2) heart sounds are expected, respectively. Prior to TAVR, there was a mid-systolic crescendo-decrescendo signal between S1 and S2, consistent with a characteristic AS murmur. Post TAVR, the S1 and S2 signals were present, but the mid-systolic signal was no longer present" said Jal Panchal, Senior Data Scientist at MindMics.
MindMics' IH technology has the potential to be a game-changer in the detection and monitoring of cardiac conditions, offering a convenient and accessible alternative to traditional methods.
About Aortic Stenosis
Aortic valve stenosis - or aortic stenosis - is a type of heart valve disease (valvular heart disease). The valve between the lower left heart chamber and the body's main artery (aorta) is narrowed and doesn't open fully. This reduces or blocks blood flow from the heart to the aorta and to the rest of the body. Treatment of aortic stenosis depends on the severity of the condition. You may need surgery to repair or replace the valve. Without treatment, severe aortic valve stenosis can lead to death.
About Transcatheter Aortic Valve Replacement (TAVR)
Transcatheter Aortic Valve Replacement (TAVR) is a minimally invasive medical procedure designed to replace the aortic valve in patients with severe aortic stenosis. Unlike traditional open-heart surgery, TAVR involves the insertion of a catheter through blood vessels, often in the leg, to guide a replacement valve into position within the diseased aortic valve. This innovative technique offers a less invasive alternative, particularly beneficial for high-risk or inoperable patients. TAVR has demonstrated shorter recovery times and reduced complications compared to open-heart surgery, making it a valuable option for individuals seeking effective treatment for aortic valve diseases.
About In-ear Infrasonic Hemodynography
In-ear infrasonic hemodynography (IH) is a novel technology for monitoring heart activity through detection of low-frequency acoustical vibrations in an occluded ear canal. Biosignals associated with the vascular hemodynamics travel within arteries, fluids, bones, and muscles in proximity to the ear canal, where they are amplified by a pressure increase of the sealed ear-canal cavity and passively detected as acoustic waveforms. The technology can be embedded into wearable devices, such as everyday in-ear headphones and hearing aids. https://rdcu.be/c16KI.
For more information: www.mindmics.com


 September 18, 2025
September 18, 2025 









