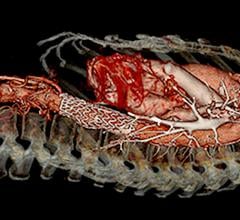
March 22, 2022 – A retrospective evaluation of 600 fenestrated-branched endovascular aortic repairs for the treatment of complex aneurysms revealed that fewer than one in five patients experienced an intraoperative adverse event (IAE).
Thoraco-abdominal aortic aneurysm repair is among the most complex and serious operations in the realm of surgery. Endovascular repair of these aneurysms is now established as a viable alternative to open surgical repair, and, according to principal author Gustavo Oderich, MD, from the University of Texas Health Science Center at Houston, “studies have demonstrated superior results to open repair.”
“However, despite many technical improvements in complex endovascular repairs, the procedure remains technically demanding with significant risks. Technical failures indeed may result in disastrous complications such as loss of a kidney, bowel or spinal ischemia.”
As reported in the March 2022 edition of the Journal of Vascular Surgery, the aim of this study was to review the incidence of IAEs and its impact on outcomes of fenestrated-branched endovascular aneurysm repair (FB-EVAR) for the treatment of complex aortic aneurysms.
The authors reported on 600 consecutive repairs performed at the Mayo Clinic between 2007 and 2019. The overall 30-day mortality was 2%. There were 122 IAEs, defined as any intraoperative complication or technical problem requiring an additional or unplanned procedure among 105 patients (18%). The most frequent events included 55 target artery, 46 access and seven graft complications.
Although IAEs did not affect patient survival (odds ratio of 1.0), suggesting the intraoperative rescue maneuvers were successful, there were more major adverse events in the IAE group, mostly due to acute kidney injury (27% versus 11%, P<.001). Risk factors for the IAEs included female sex (odds ratio 2.5), presence of target artery stenosis (odds ratio 2.0), and Crawford Extent II aneurysm (odds ratio 1.9).
“Data on the incidence of IAEs during FB-EVAR and its clinical sequelae has not been previously described in detail,” said Oderich. “This large single-center study showed that IAEs were present in 18% of patients who underwent this procedure, two-thirds of which required additional procedures to treat the complications.
“Endovascular technology continues to evolve. Novel devices have added preloaded systems, lower profile fabric, and steerable catheters and sheaths to minimize procedural difficulty.” The study also underscores the need for not only careful treatment planning, but also operators should have an armamentarium of skillsets and devices to address these IAEs.
Approximately 15,000 people in the United States are diagnosed with thoracic aortic aneurysms every year, usually people in their 70s and 80s. Still young people can develop these aneurysms, particularly if they have genetically inherited aortic conditions.
Read the full article here.


 September 18, 2025
September 18, 2025