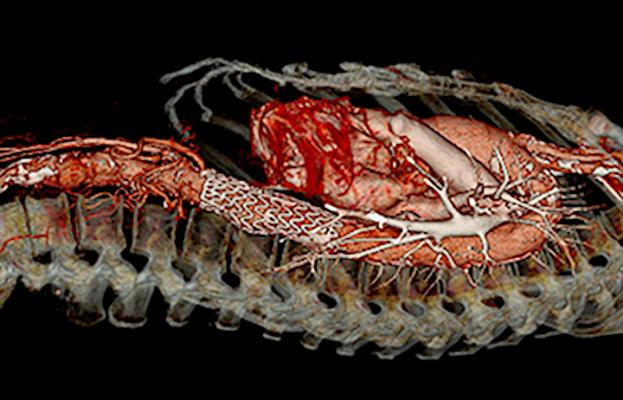
January 31, 2020 – The Society for Vascular Surgery (SVS) and Society of Thoracic Surgeons (STS) released new reporting standards to ensure patients with Type B aortic dissections (TBAD) receive appropriate treatment and care.
The care of patients with Type B dissections has evolved over time and now includes medical, surgical and endovascular therapies. Therapy is often in a multidisciplinary environment, and performed by several specialties, including vascular surgery, cardiothoracic surgery, interventional radiology and cardiology.
However, TBAD remains a life-threatening problem whose treatment has been confused by differences in nomenclature and terminology. This combined effort by the SVS and STS provides a unified consensus on reporting, nomenclature and classification of TBAD. The revision was led by Joseph Lombardi, M.D., head, division of vascular and endovascular surgery, Cooper University Healthcare, of SVS, and Chad Hughes, M.D., associate professor of surgery, Duke University, of STS.
“The new reporting standards represent the culmination of more than a year’s long effort by a writing team consisting of subject matter experts from the SVS and STS,” Hughes said. “The multidisciplinary input from both vascular and cardiothoracic surgeons is unique and has resulted in a document that will define proper reporting for this complex topic. Uniform reporting will allow standardized comparisons across series between different institutions worldwide with the goal being data that will best inform outcomes and guide best practices going forward.”
Acute aortic dissection is the most common emergency affecting the aorta, with high mortality and morbidity rates without appropriate and timely care. “With the recent blanket U.S. Federal Drug Administration (FDA) approval of endovascular stent grafting for type B aortic dissection, as well as our maturing understanding of the anatomy and pathophysiology of the disease, there has been an explosion of literature in multiple specialty journals regarding TBAD presentation, treatment and outcomes,” Lombardi said. “The purpose of this document is to provide structure to the reporting of TBAD, with particular attention to those attributes of TBAD which, based on the best available evidence to date, would appear to impact outcomes.”
The SVS/STS Reporting Standards introduce a new classification system with several new and easy-to-use features created by combining previous classification systems with the well-known anatomic zones of the aorta. “The new classification system provides an easy way to be descriptive of patients’ anatomy using language that is relevant to the way we currently treat patients,” Lombardi explained.
Another important area described is the need for a clear and consistent definition of chronicity of aortic dissections. The new standards also provide definitions of “uncomplicated” and “complicated” dissections and includes a new “high-risk” category of dissections that is defined by the presence of specific ominous clinical and radiographic features when a patient presents with a TBAD. These reporting standards also provide strict definitions of the common clinical complications of dissections and how they should be described and reported.
“The document addresses a number of limitations of the current literature by bringing consistency to all aspects of type B dissection reporting such as definitions of dissection chronicity, complicated versus uncomplicated versus high-risk dissection status, classification of dissections involving the aortic arch, which are not well described using the current Stanford and DeBakey systems, as well as a uniform definition of ‘aortic remodeling,’ among others,” Hughes said.
These are just some of the new features that will make these SVS/STS Reporting Standards an essential tool by providing a common language around dissections for clinicians, investigators, industry partners and regulatory agencies, SVS and STS representatives said.
Read the new guideline: www.jvascsurg.org/article/S0741-5214(19)32649-7/fulltext
Aortic Dissection: Management and Treatment


 November 14, 2025
November 14, 2025 









