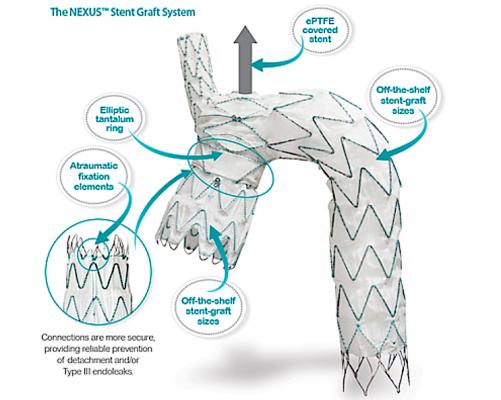
The Endospan Ltd. Nexus aortic arch stent graft is a CE mark–approved, off-the-shelf system for endovascular treatment of pathologies extending or involving the aortic arch.
November 7, 2019 — The Endospan Ltd. Nexus aortic arch stent graft is a CE mark–approved, off-the-shelf system for endovascular treatment of pathologies extending or involving the aortic arch. Two-year results from a prospective multicenter premarket study including 25 patients (mean age, 73 years) treated with Nexus were presented at the 2019 Vascular Interventional Advances (VIVA) annual meeting.
Mid-term results at a mean 25-month follow-up are stable without any material failure or stent graft issues, said presenter Daniel Clair, M.D., chair of the Department of Surgery at Palmetto Health-USC Medical Group and senior medical director for Surgical Services at Prisma Health–Midlands. However, he said more experience and longer follow-up are necessary to confirm these promising mid-term results.
Nexus is a modular stent graft introduced via a 20 French delivery system with double flushing ports, which allows efficient de-airing. The main module is deployed over an axillofemoral guidewire to extend from the brachiocephalic trunk to the descending aorta and is combined with a pre-curved ascending module that conforms to the ascending aorta.
All patients had aneurysm size >55 mm and were considered high risk for conventional open arch surgery by a multidisciplinary team. Indications for treatment were aneurysm (15 [60%] patients) or dissection (10 [40%] patients), including seven patients previously treated surgically for type A dissection. Thirteen (52%) patients had prior thoracic aortic surgery. Supra-aortic bypasses were performed prior to Nexus implantation.
Technical success was achieved for all intended Nexus introductions and deployments (100%). At 30 days, two (8%) patients died from cardiac causes and two (8%) patients showed nondisabling stroke, which resolved completely within 30 days. During a mean follow-up of 25 months, there was one additional procedure-related mortality from stroke, and one patient was converted to open surgery following retrograde type A dissection. During follow-up, aneurysm size decreased or remained stable in 96% of the patients, with no aneurysm-related deaths.
The Nexus system is the first European CE mark–approved branched stent graft for the aortic arch. Design features that assist in minimizing manipulations may explain the high technical success, low rate of neurologic complications, and durability, allowing safe endovascular repair.
Find information on all the VIVA 2019 Late-breaking Clinical Trials


 January 05, 2026
January 05, 2026 









