October 26, 2022 — Thousands of people have new hope for treatment of thoracic aortic arch disease and University Hospitals (UH) Harrington Heart & Vascular Institute is at the forefront of studying the safety and efficacy of this new procedure. A surgical team from UH is the first in Northeast Ohio to implant the NEXUS Aortic Arch Stent Graft System into a patient as part of the TRIOMPHE IDE Study.
The TRIOMPHE Study is a multi-arm, multi-center, non-randomized, prospective, clinical study to evaluate the safety and effectiveness of the NEXUS system in treating problems of the thoracic aorta that involve the aortic arch -- the segment of the aorta that helps distribute blood to the head and upper extremities. The study will enroll 100 patients. UH Harrington Heart & Vascular Institute is one of 30 participating sites in the United States and New Zealand.
“Treating patients with thoracic aortic arch disease presents many challenges,” said Jae Cho, MD, vascular surgeon, co-director of the Aortic Disease Center at UH Harrington Heart & Vascular Institute, and the Brenda and Marshall Brown Master Clinician in Vascular Innovation. “As leaders in the fields of cardiac and vascular surgery, we knew this was an important study in which our participation could make a great difference for patients. This system has the potential to create a better experience for patients and improve outcomes.”
“We are so pleased to be able to provide a treatment for our patients with aortic arch and descending aortic pathologies, including arch aneurysms and chronic dissections, that doesn’t involve a big open chest surgery,” said Yakov Elgudin, MD, PhD, cardiac surgeon at UH Harrington Heart & Vascular Institute. “Minimally invasive procedures improve the patient experience because there is generally less pain and a shorter recovery time compared to procedures where we need to open their breastbone.”
More than 120,000 patients suffer thoracic aortic arch disease every year in the USA and Europe, with only about 25 percent being diagnosed or treated. This disease causes a weakened area in the aorta and can lead to serious health risks. The weak area can cause a bulge in the portion of the aorta closest to the heart which can burst or rupture and lead to severe life-threatening internal bleeding. This portion of the aorta can also experience problems like ulcers, tearing, and leaking.
The aortic arch presents complexities for repair because of its size, shape and location. Despite major advances, open chest surgical aortic arch repair has relatively high death and complication rates, and for these reasons, many patients aren’t eligible. Historically, even minimally invasive repair to the aortic arch has proven difficult.
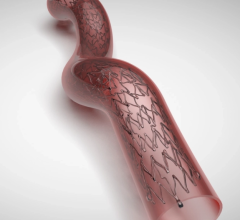
The NEXUS Aortic Arch Stent Graft System was specifically engineered for minimally invasive total arch repair to overcome the challenges of the aortic arch anatomy. NEXUS is delivered by traveling through a patient’s vascular system using fluoroscopic guidance (x-ray pictures), starting with a small incision or needle puncture near the groin. This delivery method is called an endovascular approach.
NEXUS is designed to make a minimally invasive repair possible for more patients while possibly reducing the risks of surgery. This could correlate to a reduction in both procedure time and post-operative hospital stay, as well as potentially more optimal outcomes.
“Improving the lives of our patients is what’s most important. This is why we participate in research studying the future of cardiovascular treatments and we’re hopeful to discover more successful therapies for challenging cases,” said Dr. Cho.
For more information: www.uhhospitals.org
Related content:
Nexus Arch Branch Stent Graft System Shows Positive Mid-term Results


 November 09, 2025
November 09, 2025