May 25, 2021 — iRhythm Technologies Inc. announced two new U.S. Food and Drug Administration (FDA) 510(k) clearances — ...
Patient Monitors
May 6, 2021 — The wearable cardiac devices market is set to grow from its current market was valued at more than $1.2 ...
March 24, 2021 — Endotronix Inc., a digital health and medical technology company working on advancements in treating of ...
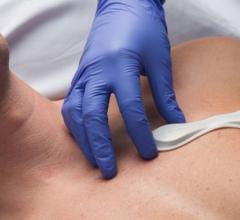
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
March 3, 2021 — AliveCor recently announced a new collaboration with AstraZeneca to research new disease management ...
February 23, 2021 — Remote cardiac monitoring vendor RhythMedix released its next-generation RhythmStar wearable device ...

The overall trend in the cardiac output monitoring market is a movement toward noninvasive or minimally monitoring ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
February 2, 2021 — Cardiac Insight, Inc. a healthcare company specializing in wearable cardiac sensors and automated ele ...
January 26, 2021 — Pedra Technology, a privately held company, announced today that the U.S. Food and Drug ...
December 22, 2020 — The U.S. Food and Drug Administration (FDA) cleared Biotronik's Vital Data Sensor, which identifies ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
November 24, 2020 — The U.S. Food and Drug Administration (FDA) has cleared AliveCor's next generation of interpretive a ...
Steven Lubitz, M.D., MPH, cardiac electrophysiologist, Massachusetts General Hospital, presented the late-breaking VITAL ...
November 16, 2020 — Atrial fibrillation (AF), an irregular heartbeat that can increase the likelihood of stroke, was ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The newer ECG devices we have now are so much less cumbersome. It’s like wearing a Band-Aid versus carrying a bulky device,” said the Greenwood, Mississippi internist. “My patients prefer the more comfortable, wire-free form factor, and the quality is as good as, or better, than the Holter,” continued Boler. “Plus, my patient compliance has increased. With the Holter, the leads sometimes come off. The patient may think the device isn’t working, so they take it off and we have to restart the process.”
September 30, 2020 — In today’s digital age, focus on digital health and the quantified self have led to the rapid rise ...
September 25, 2020 — Remote and in-hospital wearable biosensor technology company VitalConnect Inc. has started the ...
September 24, 2020 — Fitbit received 510(k) clearance from the U.S. Food and Drug Administration (FDA), as well as ...


 May 25, 2021
May 25, 2021