August 5, 2020 – NorthShore University HealthSystem , Carnegie Mellon University and physIQ are collaborating on a multi ...
Patient Monitors
July 22, 2020 — A team from Purdue University has developed self-powered wearable triboelectric nanogenerators (TENGs) ...
July 15, 2020 – Omron Healthcare Inc. and Mount Sinai Health System, New York City’s largest academic medical system ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
July 7, 2020 – Medtronic announced it received U.S. Food and Drug Administration (FDA) clearance and European CE mark ...
May 2020 — An necklace has been developed that detects abnormal heart rhythm will be showcased for the first time on EHR ...
May 5, 2020 — Wearable biosensor vendor VitalConnect Inc. was granted emergency use authorization (EUA) status by the U ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...

Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...

When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
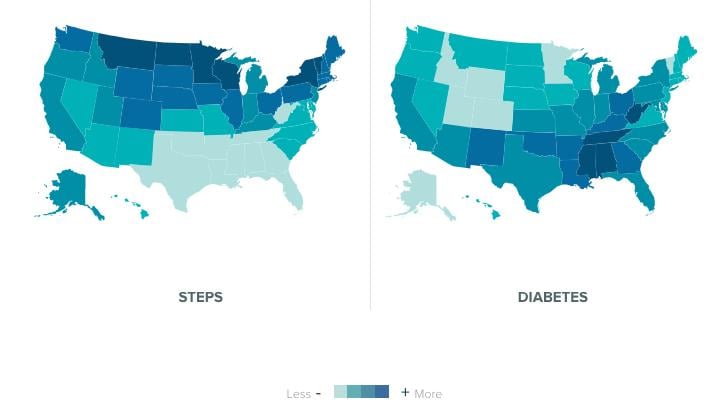
The Centers for Disease Control and Prevention (CDC) highlights physical activity as one of four key behaviors people ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...

August 15, 2019 — Bardy Diagnostics Inc. announced that HealthTech Arkansas, a healthcare accelerator and investment ...
July 31, 2019 — Silicon Valley-based digital health company Eko announced Eko Home, a new service that enables precise r ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The newer ECG devices we have now are so much less cumbersome. It’s like wearing a Band-Aid versus carrying a bulky device,” said the Greenwood, Mississippi internist. “My patients prefer the more comfortable, wire-free form factor, and the quality is as good as, or better, than the Holter,” continued Boler. “Plus, my patient compliance has increased. With the Holter, the leads sometimes come off. The patient may think the device isn’t working, so they take it off and we have to restart the process.”
June 18, 2019 – New study results validate the effectiveness of the Saranas Early Bird Bleed Monitoring System to sense ...
Khaldoun Tarakji, M.D., MPH, staff physician in the Section of Electrophysiology and Pacing in the Robert and Suzanne ...
March 26, 2019 — A toilet seat-based cardiovascular monitoring system created by a team of Rochester Institute of ...


 August 05, 2020
August 05, 2020