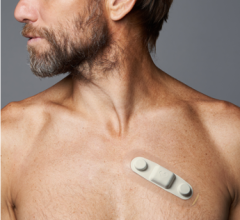
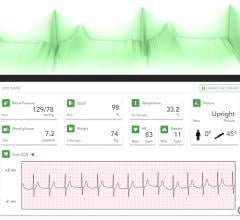
Oct. 21, 2025 — Early and accurate detection of heart rhythm problems can mean the difference between life-saving intervention and a missed opportunity, which is why new advances in cardiac ...
ECG Wireless Remote Access
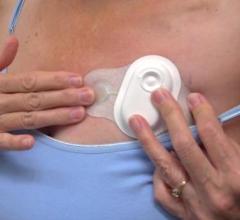
This channel includes news and new technology innovations for ECG monitoring services that allow physicians to measure a patient's heart rhythm over a period of time using wireless remote access.
Oct. 21, 2025 — Early and accurate detection of heart rhythm problems can mean the difference between life-saving ...
July 22, 2025 — HeartBeam, Inc., a medical technology company focused on transforming cardiac care by providing powerful ...
May 31, 2024 — InfoBionic.ai and HBox.ai announced a groundbreaking partnership that brings together the leading AI ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
May 10, 2024 — With cardiovascular disease being a leading cause of mortality worldwide, Vivalink, a leading provider of ...
September 27, 2023 — HeartBeam, Inc., a Santa Clara, CA-based cardiac technology company, has appointed Richa Gujarati ...
June 21, 2023 — Qardio, a leading innovator in healthcare technology, has unveiled its groundbreaking Livestream ...
Improved short-term monitoring methods for patients with stroke risk can increase early detection of atrial fibrillation ...
December 8, 2022 — Heart disease is the number one killer in the U.S., and high-fidelity monitoring is the beginning of ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
Improved short-term monitoring methods for patients with stroke risk can increase early detection of atrial fibrillation ...
Improved short-term monitoring methods for patients with stroke risk can increase early detection of atrial fibrillation ...
January 4, 2022 – Remote patient monitoring and cardiac data management solutions vendor Implicity received U.S. Food ...
December 14, 2021 – The National Capital Consortium for Pediatric Device Innovation (NCC-PDI) announces five awardees ...

Improved short-term monitoring methods for patients with stroke risk can increase early detection of atrial fibrillation ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
November 22, 2021 — Cardiologs, a leader in artificial intelligence (AI) cardiology diagnostics, announced the results ...
November 1, 2021 — Health AI company Vagus.co, based in Cambridge, U.K., has launched a 30-second breathing test for ...
October 15, 2021 — Biotricity Inc. announced the release of Biocare Cardiac, a personal, cardiac health application for ...




 October 21, 2025
October 21, 2025