May 25, 2021 — iRhythm Technologies Inc. announced two new U.S. Food and Drug Administration (FDA) 510(k) clearances — one for a new and improved design of its flagship Zio wearable cardiac monitor, and a second for updated artificial intelligence (AI) capabilities.
The new Zio monitor is designed to significantly improve patient comfort, while the advancements to its AI capabilities will further improve rhythm and beat diagnostic accuracy.
“By improving patient comfort and experience we can continue to maximize patient compliance, which is an essential element for collecting high-quality data for analysis," explained Mike Coyle, CEO of iRhythm. "And with the power of our next generation AI, we can help physicians better identify, diagnose and manage a wide array of significant arrhythmias, including atrial fibrillation.”
New Zio Monitor Has Increased Comfort
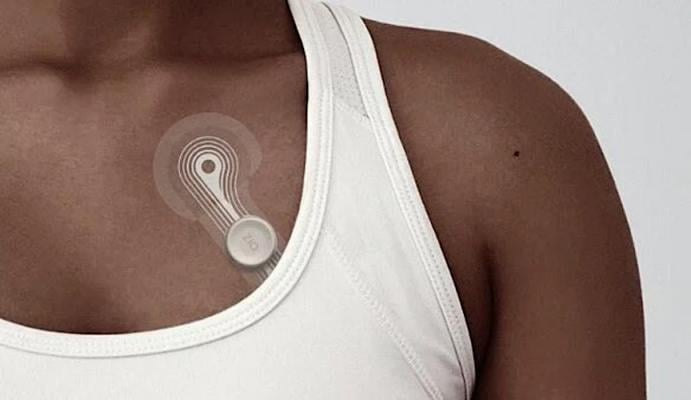
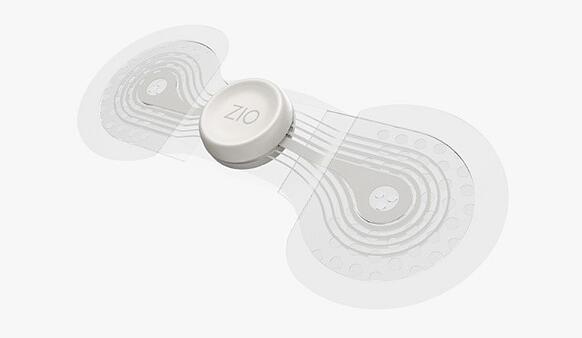 The new Zio monitor is designed to be effortless to wear with increased adherence and better patient comfort. It is small enough for patients to forget they are wearing it through exercise, showering and sleeping. The new design is more than 50% lighter than the current generation, and includes a new breathable and waterproof outer layer. It also has an improved "stay-put" adhesive and a more flexible design for a secure attachment. The company said these refinements will allow for a more comfortable wear and, therefore, more complete, accurate diagnostic data.
The new Zio monitor is designed to be effortless to wear with increased adherence and better patient comfort. It is small enough for patients to forget they are wearing it through exercise, showering and sleeping. The new design is more than 50% lighter than the current generation, and includes a new breathable and waterproof outer layer. It also has an improved "stay-put" adhesive and a more flexible design for a secure attachment. The company said these refinements will allow for a more comfortable wear and, therefore, more complete, accurate diagnostic data.
“iRhythm started with the patient in mind first,” said Judy Lenane, RN, chief clinical officer and executive vice president of products at iRhythm. “We asked ourselves what it would take to give patients the best, most comfortable experience, and designed it from there. We’re excited to offer more patients around the world access, as well as an improved patient experience, which will enable far more insight into the data.”
Enhanced AI Algorithm More Accurately Detects Arrhythmias
iRhythm has made significant system improvements to its deep-learned AI algorithm. The company has collected over 750 million hours of curated heartbeat data, creating the largest repository of labelled ECG patient data in the world. This allows for expanded training of the algorithm across a larger database, resulting in enhanced AI diagnostic accuracy and better quality assurance. On top of the record expansion, iRhythm now has a much more technologically advanced AI backbone – moving from machine-learned to deep-learned capabilities. With AI, the average rhythm detection sensitivity of iRhythm’s detection algorithm has improved by 21% since its creation in 2010.
iRhythm was the first cardiac monitor to use a FDA-cleared, deep-learned algorithms for classifying and characterizing diverse heart rhythms. iRhythm is now using AI to detect beats, beat types and heart rates – on top of the deep-learned rhythm detection capability introduced and clinically validated in 2019. This allows for AI-based detection of heart activity at a much more granular level, with an enhanced level of accuracy.
The updated AI capabilities will be introduced later this quarter for the U.S.-based Zio Service, while the new Zio monitor platform will begin limited release later this year.


 November 12, 2025
November 12, 2025 









