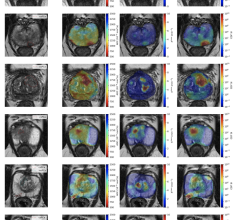
July 15, 2020 – Omron Healthcare Inc. and Mount Sinai Health System, New York City’s largest academic medical system, have teamed to offer patients the new VitalSight home blood pressure monitoring solution. Mount Sinai recently rolled out the VitalSight program to support the needs of their patients with hypertension.
The effort to provide VitalSight to patients is led by Rob Fields, M.D., SVP and chief medical officer for population health at Mount Sinai, and a team of clinical pharmacists, who are responsible for the day-to-day management of the program and coordination with physicians to ensure that patients receive individualized care.
“The ability to monitor patients at home during the pandemic – and on an ongoing basis – is critical. Our collaboration with Omron Healthcare helps make patients active participants in their own healthcare and extends the reach of clinicians, who receive a continuous stream of their patients’ real-time health data so that they can proactively intervene as necessary,” Fields said. “Additionally, we are focusing first on our most vulnerable patients, who bear the consequences of disparities in care – in part, due to lack of technology access. This program requires no technology and comes at no cost for the device, with little-to-no cost for service.”
“We’re excited to collaborate with such a prestigious healthcare institution as Mount Sinai, who played an instrumental role in shaping the value of VitalSight from early in its development. Now, they are leading the way for patients to use VitalSight from the privacy of their home, while staying closely connected to their physician,” explained Omron Healthcare President and CEO Ranndy Kellogg.
VitalSight is the newest addition to the line of Omron Healthcare home blood pressure monitors, which are among the top doctor and pharmacist recommended systems.[1,2] The VitalSight kit complements Mount Sinai’s commitment to remotely monitor patients as part of its recent telehealth initiative, especially as providers care for COVID-19 patients who are recovering at home.
VitalSight is a HIPAA-compliant, Medicare-reimbursable home blood pressure monitoring solution that generally comes at no cost to the patient, depending on their coverage. The kit typically includes a digital blood pressure monitor with cuff, weight scale and digital medication tracker, as well as a data hub. Exact devices may vary based on what the physician deems appropriate for each patient’s hypertension monitoring needs. VitalSight directly links to a physician’s electronic medical record (EMR), and is compatible with leading systems. Patients measure their blood pressure, weigh themselves and continue to take their medication as ordered by their physician. Securely encrypted data is automatically sent to the doctor’s EMR in real time, where it’s stored for reference unless a health concern is detected, in which case the physician’s office is alerted.
For more information: www.omronhealthcare.com
References:
1. Frost & Sullivan Survey, Blood pressure clinician perception tracker surveys. 17 July 2019.

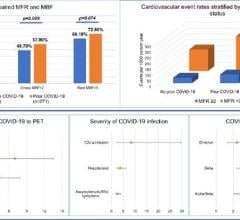
 March 20, 2024
March 20, 2024