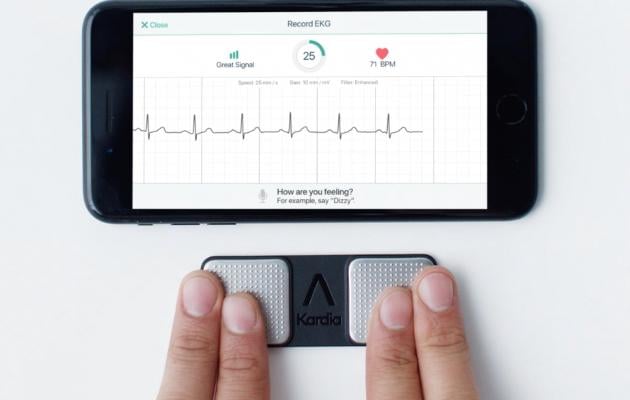
Alivecor's pocket ECG system allows consumers or cardiologists to record a single lead ECG strip on a smartphone. AI algorithms can determine if their ECG is normal or abnormal and identify the arrhythmia.
March 3, 2021 — AliveCor recently announced a new collaboration with AstraZeneca to research new disease management solutions in cardiovascular, renal and metabolism (CVRM) therapeutic areas. The collaboration will translate AliveCor’s potassium detection technology and science, which enables potassium measurement outside of blood draws, into real-world disease management applications and solutions.
The cross-industry collaboration expands the research and development of AliveCor’s Kardia-K artificial intelligence (AI) platform, which received FDA breakthrough device designation status to screen for elevated levels of blood potassium. Kardia-K is being built using AliveCor’s proprietary deep neural network and analyzes electrocardiograms (ECGs) to measure a patient’s potassium levels without requiring any blood from the patient.
AliveCor’s neural network was trained in collaboration with Mayo Clinic using more than 1.5 million ECGs and was validated on approximately 62,000 ECGs. The research was published in JAMA Cardiology in April 2019.
“By collaborating across industries, AliveCor is leading the way in the development of non- invasive, more accessible medical solutions for patients and health organizations worldwide,” said Aman Bhatti, head of biopharma partnerships at AliveCor. “Our collaboration with AstraZeneca exemplifies how pharmaceutical and digital health companies can work together to drive the future of medicine.”
For the nearly 30 million U.S. adults with chronic kidney disease, the one-day likelihood of a fatality is 3 to 13 times higher if potassium is elevated. The current standard practice for measuring potassium levels is a blood test, which is invasive, inconvenient, and poses safety risks for patients during the pandemic. A remote, easy-to-use potassium test could help track for increased potassium levels in those patients, as well as the 500,000 Americans with end stage kidney disease and on dialysis, where high potassium may contribute to up to 40% of fatalities.
The collaboration seeks to improve the delivery of life-changing medicines that are fueling growth in the industry and providing value to patients and society. Its initiative accelerates AliveCor’s work with key players in the BioPharma industry and demonstrates diverse interest in AliveCor’s technology, from clinical research organizations to health systems to pharmaceutical companies’ digital therapeutic programs.
AliveCor is transforming cardiology care using deep learning and smartphone enabled devices. The FDA-cleared KardiaMobile device is the most clinically validated personal ECG solution in the world. KardiaMobile provides instant detection of atrial fibrillation, bradycardia, tachycardia, sinus rhythm with supraventricular ectopy, sinus rhythm with premature ventricular contractions, sinus rhythm with wide QRS and normal heart rhythm in an ECG. Kardia is the first AI-enabled platform to aid patients and clinicians in the early detection of atrial fibrillation.
AliveCor's enterprise platform allows third party providers to manage their patients’ and customers’ heart conditions simply and profitably using state-of-the-art tools that provide easy front-end and back-end integration to AliveCor technologies. AliveCor protects its customers with stringent data security and compliance practices, achieving HIPAA compliance and SOC2 Type 1 and Type 2 attestations. AliveCor is a privately-held company headquartered in Mountain View, Calif. “Consumer” or “personal” ECGs are ECG devices available for direct sale to consumers.
For more information: alivecor.com


 November 12, 2025
November 12, 2025 









