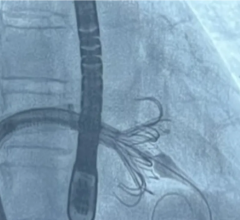
July 3, 2024 — Jon Kobashigawa, MD, director of the Heart Transplant Program in the Department of Cardiology in the ...
Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
July 2, 2024 — The U.S. Food and Drug Administration (FDA) announced that Abbott has issued a correction for its ...
June 27, 2024 — The sheer scale of undiagnosed heart valve disease in our aging population has been revealed for the ...
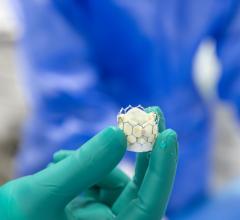
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
June 24, 2024 — Aakriti Gupta, MD, and Michelle Kittleson, MD, PhD, cardiologists in the Smidt Heart Institute at Cedars ...
June 21, 2024 — UC San Francisco interventional cardiologists and interventional echocardiographers recently performed ...
June 20, 2024 — atHeart Medical, a medical device company establishing a new standard of care for atrial septal defects ...
June 13, 2024 — Medtronic plc, a global leader in healthcare technology, today announced the launch of its latest ...
June 13, 2024 — Xeltis, a leading developer of transformative implants that enable the natural creation of living and ...
On July 16, Diagnostic and Interventional Cardiology will present a webinar on "Maximizing Structural Heart Workflows ...
June 10, 2024 — Atrium Health Sanger Heart & Vascular Institute has successfully completed the first commercial ...
June 7, 2024 — Medtronic today announced new data from the CoreValve Evolut Clinical Program, reinforcing the positive ...
June 5, 2024 — Edwards Lifesciences announced it has entered into a definitive agreement to sell its Critical Care ...

Here is a look at the Top 10 pieces of content viewers were reading during the month of May.
1. American College of ...
May 15, 2024 — A new study demonstrated parity between a minimally invasive procedure to replace the aortic valve in the ...
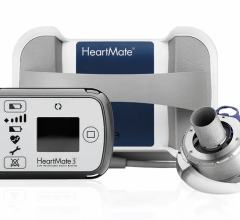
May 15, 2024 — The U.S. Food and Drug Administration (FDA) announced that Abbott is recalling the HeartMate 3 LVAS by ...


 July 03, 2024
July 03, 2024