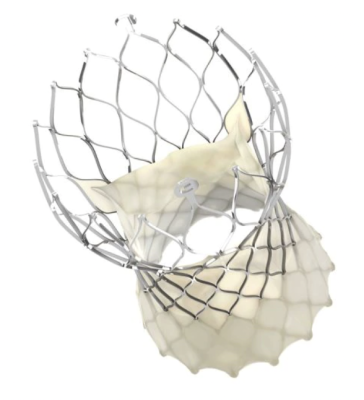
September 17, 2022 — Medtronic today announced findings from its Pathological Study of Hypo-Attenuated Leaflet Thickening (HALT) in Self-Expanding Transcatheter Aortic Valves, as part of the CoreValve Evolut Program. The study is the first to compare microCT and histology findings of valve leaflet thickening, the results of which may provide clinical insights for long-term durability of transcatheter aortic valves (TAVs) and treatment of HALT. The findings were presented as Late-Breaking Clinical Science in Structural Heart Disease at the 34th Transcatheter Cardiovascular Therapeutics (TCT) conference, the annual scientific symposium of the Cardiovascular Research Foundation.
HALT is observed on CT imaging and indicates possible formation of a blood clot on prosthetic heart valve leaflets, potentially affecting their ability to open and close freely. HALT may occur in at least10% of TAVR patients and can impact valve deterioration.1 While HALT can typically be treated with oral anticoagulants (OAC), it is sometimes resistant to drug therapy. 2,3
The study evaluated the extent of pathologic changes of valve thrombosis, neointimal thickening, inflammation, and calcification over time in 110 TAVs explanted at surgery or autopsy. The explanted valves were collected from 11 clinical studies that included more than 7,500 participants. Additionally, the study also evaluated areas of leaflet thickening by two methodologies, microCT and histology, to assess the prevalence, length, and underlying cellular composition of these areas.
Although clinical thrombosis rates are extremely low in CoreValve/Evolut clinical trials of self-expanding TAVs (0%–1.3%), the study found that approximately 45% of leaflets in explanted valves showed at least some degree of leaflet thickening; the prevalence was comparable with microCT and histology assessment. Histological scores by implant duration showed there was no change in thrombus and inflammation scores over time, however neointima, structural changes, and calcification scores increased with greater implant duration. Thrombi begin organizing after 30 days and by one year had a more organized morphology that may be resistant to treatment.
“As TAVR continues to grow as a treatment option for aortic stenosis, identifying pathological changes to TAVs over time is critical for understanding their long-term durability,” said Nina Goodheart, Senior Vice President and President of the Structural Heart & Aortic business, which is part of the Cardiovascular Portfolio at Medtronic. “Today’s results offer additional clinical evidence for our CoreValve Evolut Program to better understand the importance of early treatment when HALT is detected.”
“These findings help to address the need to better understand HALT development and treatment, particularly in cases where OAC therapy fails. Further, we believe these findings underscore the need for early detection and treatment within the first year after implantation. This may help to optimize outcomes for patients receiving transcatheter aortic valves.” said Yu Sato, MD, CVPath Institute study lead.
The Medtronic CoreValve Evolut R, CoreValve Evolut PRO, and Evolut PRO+ systems are indicated for relief of aortic stenosis in patients with symptomatic heart disease due to severe native calcific aortic stenosis who are judged by a heart team, including a cardiac surgeon, to be appropriate for the transcatheter heart valve replacement therapy.
For more information: www.medtronic.com
More TCT22 conference coverage
References:
1Hein M, Schoechlin S, Schulz U, et al. Long-Term Follow-Up of Hypoattenuated Leaflet Thickening After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol Intv. 2022 Jun, 15 (11) 1113–1122. https://doi.org/10.1016/j.jcin.2022.04.018
2De Backer O, et al. NEJM. 2020;382:130-139.
3Blanke P, et al. JACC. 2020;75:2430-2442.


 October 31, 2025
October 31, 2025 









