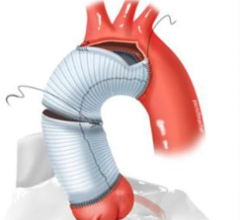
May 27, 2025 — Abbott has announced the U.S. Food and Drug Administration (FDA) has approved the company's Tendyne ...
Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
May 2, 2025 – New analysis from the EARLY TAVR trial showed patients between the age of 65 and 70 years old derived the ...
April 28, 2025 — The Society of Thoracic Surgeons (STS) has launched its latest surgical risk calculator designed for ...
March 30, 2025 — Medtronic has announced late-breaking data on five-year outcomes from the Evolut Low Risk Trial. Data ...
March 17, 2025 — Being born with a heart defect may be associated with an increased cancer risk for babies and their ...
Feb. 22, 2025 — More than 60,000 people die from heart valve disease (HVD) in the U.S. each year, according to the ...
Feb. 17, 2025 — The International Consortium for Health Outcomes Measurement (ICHOM) has developed a globally inclusive ...
Feb. 13, 2025 — Research from Cedars-Sinai investigators and collaborators at other leading medical institutions is ...
Jan. 25, 2025—A new study presented at the 2025 Society of Thoracic Surgeons (STS) Annual Meeting reveals that ...
Jan. 6, 2025 — Medtronic plc has announced it received CE (Conformité Européenne) Mark for the Harmony Transcatheter ...
Sept. 1, 2024 — Researchers at UTHealth Houston have identified genetic variants linked to a rare form of bicuspid ...
July 29, 2024 — Edwards Lifesciences announced investments that reflect the company’s deep commitment to advancing ...
On July 16, Diagnostic and Interventional Cardiology presented a webinar on "Maximizing Structural Heart Workflows ...
July 15, 2024 — Edwards Lifesciences announced it has exercised its option to acquire Innovalve Bio Medical Ltd., an ...
July 9, 2024 — Disparities in cardiovascular disease outcomes between urban and rural areas continue to widen, yet ...


 May 28, 2025
May 28, 2025