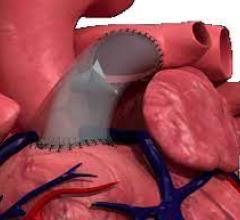
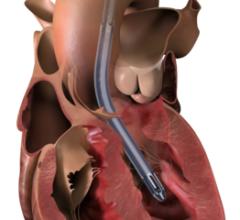
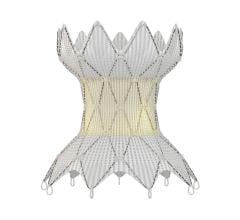
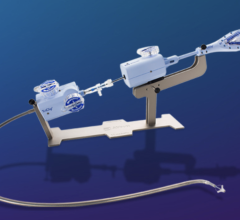
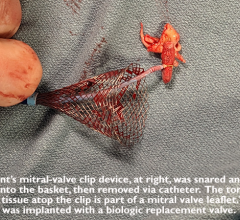
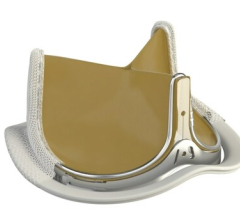
June 22, 2023 — Lankenau Medical Center, part of Main Line Health, has performed the first minimally invasive catheter ...
Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
June 13, 2023 — PECA Labs, a medical device company reimagining vascular grafts and valves with durable polymeric ...
June 9, 2023 — Dasi Simulations announced it received FDA clearance for its artificial intelligence model for ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
June 6, 2023 — According to a news release issued by the U.S. Food and Drug Administration (FDA), Abiomed has recalled ...
June 5, 2023 — Long-awaited outcomes data of transcatheter edge-to-edge procedures to repair patients’ leaky mitral ...
May 25, 2023 — Boston Scientific Corporation announced data supporting use of the company's key electrophysiology and ...
May 22, 2023 — Boston Scientific Corporation announced data supporting use of the company's key electrophysiology and ...
May 19, 2023 — Abbott announced late-breaking data that add to the body of clinical evidence supporting the benefits of ...
May 17, 2023 — Edwards Lifesciences announced that new data from the Benchmark Registry in Europe demonstrated the ...
March 16, 2023 — Cardiologists at the UW Medicine Heart Institute recently performed a first-in-the-world procedure ...
May 16, 2023 — Henry Ford Health Interventional cardiologists William O’Neill, M.D., and Khaldoon Alaswad, M.D., took a ...
May 15, 2023 — The Cardiovascular Research Foundation (CRF) announced that TVT: The Structural Heart Summit will feature ...
May 9, 2023 — Edwards Lifesciences announced new data from the COMMENCE aortic trial, demonstrating low rates of structu ...
April 25, 2023 — FastWave Medical, a privately-held company founded by Big Sky Biomedical partners, announces the ...


 June 22, 2023
June 22, 2023