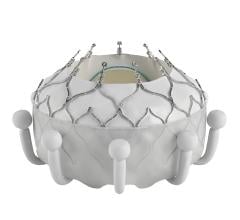
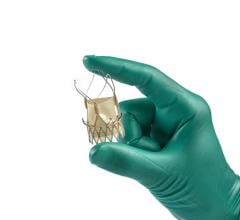
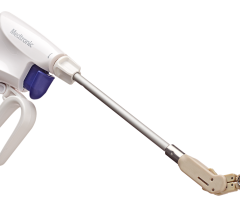
February 9, 2024 — Physicians in the Smidt Heart Institute at Cedars-Sinai have achieved two significant firsts ...
Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
February 2, 2024 — Edwards Lifesciences Corporation announced the company’s EVOQUE tricuspid valve replacement system is ...

It is a new year with lots of new content! Here is a look at the most-read content during the month of January on dicard ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
January 29, 2024 — Despite national guidelines recommending surgical aortic valve replacement (SAVR) for patients under ...
You may not know Carol Barr, but in the future, she could save your life.
Barr’s death at 39 from sudden cardiac ...
January 8, 2024 — University Hospitals (UH) Harrington Heart & Vascular Institute recently became the first center in ...
January 5, 2024 — Medtronic, a global leader in healthcare technology, today announced it has received CE (Conformité ...
January 4, 2024 — Laguna Tech USA, a privately-held medical technology company dedicated to innovations in structural ...
January 4, 2024 — Findings from a published case series research letter by the Henry Ford Health Structural Heart ...
December 22, 2023 — TRiCares SAS (“TRiCares”), a privately held pioneer in the field of minimally invasive treatment of ...
December 20, 2023 — Jason R. McCarthy, Ph.D., associate professor of biomedical research and translational medicine and ...
December 18, 2023 — Death rates related to infective endocarditis declined in most adults across the U.S. within the ...
December 12, 2023 — Think of them as the Energizer Bunnies of the heart, tiny natural batteries that keep this vital ...
December 12, 2023 — Patients who received the anticoagulant drug warfarin after bioprosthetic aortic valve replacement h ...
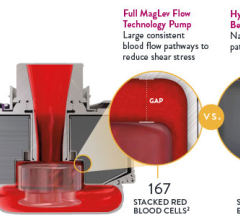
November 20, 2023 — Abbott announced new late-breaking data that show advanced heart failure patients living with its He ...


 February 09, 2024
February 09, 2024