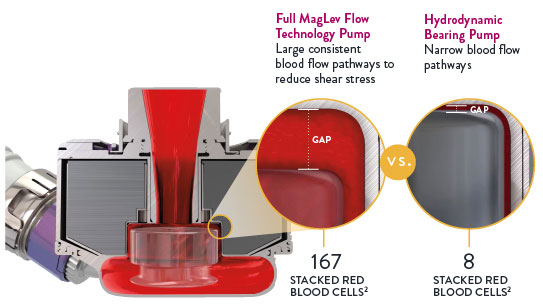
November 20, 2023 — Abbott announced new late-breaking data that show advanced heart failure patients living with its HeartMate 3 heart pump who didn't receive aspirin as part of their blood-thinning medication regimen experienced fewer complications from bleeding and were associated with reduced hospital visits compared to patients who took aspirin daily following their implant. The data from the ARIES trial is the first to potentially shift how physicians manage their patients living with a HeartMate 3 heart pump. ARIES marks the first international, placebo-controlled, randomized clinical study to assess whether the absence of aspirin is safe and decreases bleeding in people with the HeartMate 3 left ventricular assist device (LVAD, or heart pump).
The data were presented during a late-breaking presentation at the 2023 American Heart Association's Scientific Sessions in Philadelphia and simultaneously published in The Journal of the American Medical Association.
Heart Pump Patients See Benefit in Aspirin-Free Regimen
The ARIES trial studied more than 600 patients and found that HeartMate 3 patients who didn't receive aspirin but continued using the standard post-implant vitamin-K antagonist (VKA) treatment regimen met the primary endpoint by showing non-inferiority of no aspirin to aspirin. The HeartMate 3 patients who did not take aspirin spent 47% fewer days in the hospital due to a nearly 40% decrease in bleeding events compared to patients who continued to take aspirin daily.
While the HeartMate 3 is associated with lower rates of complications compared to previous generation heart pumps, bleeding remains a leading cause for rehospitalizations. The ARIES trial demonstrated a reduction in bleeding events in patients without aspirin, which could lead to an important change in management of patients with a HeartMate 3.
"The ARIES study moves the needle forward in improving the journey of advanced heart failure patients with a marked improvement in bleeding events, healthcare resource use and cost-savings by a simple decision to avoid the use of aspirin," said Mandeep R. Mehra, M.D., executive director of the Center for Advanced Heart Disease and the William Harvey Distinguished Chair at Brigham and Women's Hospital in Boston, Mass. "The data is so compelling that the magnitude of benefit observed in avoiding aspirin is similar to the impact of introducing a new device to the market."
Aspirin-Free Regimen Reduces Costs for HeartMate 3 Patients
In addition to finding reductions in bleeding and hospital visits for bleeding complications, the ARIES trial also revealed cost-savings for heart failure patients who did not take aspirin following implant of an Abbott HeartMate 3 pump. One year after receiving the device, there was a 41% reduction in estimated costs related to bleeding events. The data also found this same group had no elevated risk in developing thrombosis (a blood clot that increases the risk of stroke).
"There is a general consensus within the medical community that aspirin use should be a mandatory part of the treatment regimen for heart failure patients living with an LVAD; however, those assumptions were largely driven by observational data that have rarely been challenged," said Robert Kormos, M.D., divisional vice president, global medical affairs, Abbott's heart failure business. "The ARIES trial estimates that for every 100 people with the HeartMate 3, not taking aspirin prevents nearly 15 major bleeding events within their first year with the device. That equates to many more moments these patients can spend with their loved ones living a fuller life."
About the ARIES Trial
The ARIES trial was an international, randomized study of either aspirin (100mg/day) or placebo with VKA therapy in advanced heart failure patients with Abbott's HeartMate 3 LVAD. The study (July 2020 – September 2022) was conducted across 51 centers and included over 600 patients in the United States, Canada, the United Kingdom, France, Italy, Austria, Czech Republic, Kazakhstan and Australia. ARIES met its primary endpoint which found that for patients with the HeartMate 3, an aspirin-free medication regimen is non-inferior to an anti-thrombotic regimen that includes aspirin. The trial is part of Abbott's continued commitment to investments in heart failure and clinical science innovation to further improve the lives of people with advanced heart failure.
"Abbott has focused its investments on ways that we can continue to improve the outcomes for the thousands of patients a year who are eligible to receive a heart pump," said Keith Boettiger, vice president, Abbott's heart failure business. "With the ARIES trial, we've identified an important new approach to patient management that doctors can consider to reduce bleeding risk for their patients and make LVAD therapy more accessible to patients who need this life-saving device."
Indications and Important Safety Information:
The HeartMate 3 Left Ventricular Assist System (LVAS) is indicated for providing short- and long-term mechanical circulatory support (e.g., as bridge to transplant or myocardial recovery, or destination therapy) in adult and pediatric patients with advanced refractory left ventricular heart failure and with an appropriate body surface area. It is contraindicated for patients who cannot tolerate, or who are allergic to, anticoagulation therapy.
For additional U.S. important safety information for the Abbott HeartMate 3, visit: HeartMate 3 LVAD Indications, Safety and Warnings | Abbott (cardiovascular.abbott).
The ARIES HeartMate 3 clinical study evaluated a new clinical approach to patient management, which has not been reviewed by the U.S. Food and Drug Administration (FDA).
Labeling changes related to the study's anticoagulation regimen have not been approved by the FDA at this time.
For more information: www.abbott.com
Related content:
Effectively Treating Heart Failure: New Technology on the Horizon
Abbott Announces Future of Biowearables at Consumer Electronics Show
First Woman in World Receives New Type of Artificial Heart
FDA Clears HeartMate 3 for Heart Failure Patients Not Eligible for Transplant
Related Content on the HeartMate III:
Abbott Receives FDA Approval for HeartMate 3 Left Ventricular Assist System
VIDEO: Therapies for Advanced Heart Failure — interview with David Lanfear M.D., FACC, head of advanced heart failure and cardiac transplantation, Henry Ford Hospital, Detroit
Leading Cardiovascular Societies Release New Guidance on Use of Heart Pumps


 February 03, 2026
February 03, 2026 









