November 20, 2023 — Student engineers from the University of Bath are on top of the world after winning an international ...
Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
November 10, 2023 — BiVACOR, a clinical-stage medical device company developing the Total Artificial Heart (TAH), has ...
October 31, 2023 — Edwards Lifesciences Corporation announced one-year results from CLASP IID, the first randomized ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
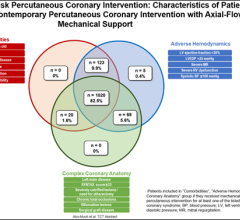
October 26, 2023 — Abbott announced data from late-breaking presentations showing the impact of its minimally invasive ...
October 26, 2023 — The Society of Thoracic Surgeons and the European Association for Cardiothoracic Surgeons have issued ...
October 25, 2023 — Findings from a trial led by Cleveland Clinic show that patients with atrial fibrillation undergoing ...
October 19, 2023 — The Dib UltraNav Transseptal Catheter System, which houses a needle and ultrasound in one system for ...
October 19, 2023 — Conformal Medical, Inc. announced today the one-year results from the company's CONFORMAL Early ...
October 19, 2023 — Edwards Lifesciences Corporation announced the company's EVOQUE tricuspid valve replacement system ...
October 17, 2023 — The Patel Children's Heart Institute at St. Joseph's Children's Hospital achieved a milestone ...
October 12, 2023 — Franklin Mountain Medical announced today that the Dib UltraNav Transseptal Catheter System, which ...
October 5, 2023 — Mayo Clinic researchers have developed a calculation that can help identify moderate aortic stenosis p ...
October 5, 2023 — A newly-issued "Scientific Statement on Durable Mechanical Circulatory Support" in the October issue ...


 November 20, 2023
November 20, 2023