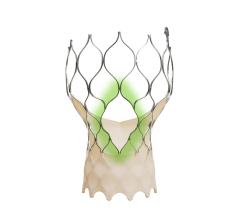
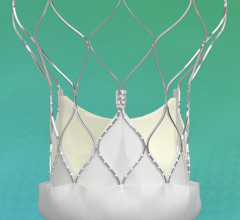
April 2, 2024 — Abbott announced that the U.S. Food and Drug Administration (FDA) approved the company's first-of-its ...
Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
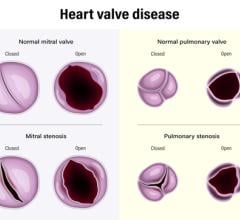
April 1, 2024 — Roughly 25,000 Americans die each year from valvular heart disease, but researchers from Rutgers Health ...
March 28, 2024 — Medtronic plc, a global leader in healthcare technology, announced that the United States Food and Drug ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
March 25, 2024 — In a groundbreaking medical advancement, three esteemed cardiologists from CLS Health, Dr. Bahaeddin ...
March 21, 2024 — The U.S. Food and Drug Administration (FDA) announced that Abiomed is recalling the Instructions for ...
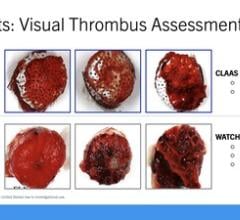
March 18, 2024 — Conformal Medical, Inc. announced that the CLAAS System was featured in a podium presentation at the Ca ...
March 12, 2024 — Medtronic plc, a global leader in healthcare technology, today announced two late-breaking data ...

February was a short but busy month in the diagnostic and interventional cardiology world, with a lot of news being ...
February 26, 2024 — Hackensack Meridian Jersey Shore University Medical Center and Hackensack University Medical Center ...

The DAIC team has learned of the passing of Alain Cribier, MD, FACC, heralded as the man who pioneered the first transca ...
PLEASE NOTE: This webinar has been postponed to a later date.
A new date will be posted in the coming days.
...
February 21, 2024 — An 80-year-old woman from Frankenmuth, Michigan is the first Henry Ford Health structural heart ...
February 20, 2024 — As New Jersey’s leading provider of high-quality cardiac procedures and diagnostic testing and an ...
February 15, 2024 — Abbott announced that the Circulatory System Devices Panel of the Medical Devices Advisory Committee ...


 April 02, 2024
April 02, 2024