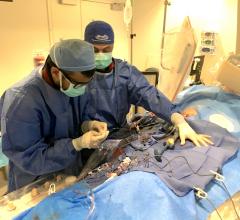
Henry Ford Health's Center for Structural Heart Disease team delivered its first in Michigan FDA-approved VOQUE Transcatheter Tricuspid Valve Replacement System to a heart failure patient on February 13, 2024 at Henry Ford Hospital in Detroit. Image courtesy of Henry Ford Health
February 21, 2024 — An 80-year-old woman from Frankenmuth, Michigan is the first Henry Ford Health structural heart disease patient—and the first ever in Michigan—to receive a new transcatheter heart valve device for treating severe tricuspid regurgitation. She is only the third patient in the U.S. to receive the device.
The team at Henry Ford’s Center for Structural Heart Disease performed the procedure at Henry Ford Hospital. The patient expressed her appreciation for her medical team while recovering at the hospital on Valentine’s Day, a day after receiving the new valve, saying “it worked out wonderfully.”
The patient, who has asked for privacy as she recovers, was selected to receive the new technology after being part of a clinical trial that helped to bring about its commercial use approval from the Food & Drug Administration (FDA) on February 1 for the breakthrough transcatheter therapy that is delivered to the heart through a blood vessel in the leg.
“This is really the first valve that can be delivered via catheter where previously open-heart surgery was the only option for patients healthy enough to undergo the procedure,” said Brian O’Neill, M.D., director of Interventional and Structural Heart Research at Henry Ford Hospital.
People with severe tricuspid regurgitation may feel unusually tired and experience shortness of breath especially during physical activity. Other symptoms include swelling in the abdomen, legs or neck veins.
“Patients with this severe condition also have increased mortality and may experience more frequent heart failure hospitalizations,” said Dr. O’Neill.
The Edwards EVOQUE Tricuspid Valve Replacement System is an artificial heart valve developed to treat patients suffering from severely leaky tricuspid valves, a condition known as tricuspid regurgitation. The condition is often caused by an enlarged heart or the patient’s native damaged valve flaps. Candidates for the new device include those who continue to experience symptoms despite being on heart failure medications.
According to published studies and papers, it is estimated that 1.5 million Americans have moderate to severe tricuspid regurgitation. However, only about 8,000 tricuspid valve surgeries are performed annually. Less than 5 percent of all eligible patients can be treated by traditional open-heart surgery.
“There are many reasons for the low number of eligible patients,” said Dr. O’Neill, “The main reason is that many patients are not deemed good candidates by their cardiologists or cardiothoracic surgeons to undergo open heart surgery.”
He added that this was the impetus to develop new transcatheter technologies that would replace open heart surgery and allow more patients living with severe tricuspid regurgitation the opportunity to receive possibly life-changing therapies.
Henry Ford Hospital was one of the first hospitals in the country that participated in the early feasibility study in 2020 dubbed TRISCEND I and the subsequent pivotal trial TRISCEND II that led to the approval of the new device. Henry Ford was one of the top 10 for patient participation nationally for these trials.
“We had the opportunity to treat patients as part of the clinical trial and we are excited that this new technology can now be offered to more patients,” said Dr. O’Neill.
Henry Ford’s Structural Heart Disease program had many patients being evaluated as possible candidates for a new valve when the FDA announcement was made. “Now that the trial is over and the device is commercially available, we are evaluating our trial patients to see if they fit the criteria for receiving the new device,” said Dr. O’Neill, who added that the majority of severe tricuspid valve regurgitation patients under the care of Henry Ford Hospital will be able to be treated with the new valve.
As a participating trial site, heart patients who come to Henry Ford Health for an evaluation with a Structural Heart Disease cardiologist will have the earliest access possible for this new treatment. Patients can also visit the medical technology company website to learn more and begin the evaluation process.
William O’Neill, M.D., medical director emeritus of Structural Heart Disease at Henry Ford Health, helped bring the first EVOQUE device to Henry Ford years earlier, which paved the way for the new valve to receive recent FDA approval. That approval will now pave the way for many more patients with leaky tricuspid valves to receive potentially life-changing help.
“We were part of the research team that tested the valve in clinical trials and have been testing different ways of treating the tricuspid valve for seven years, so it’s wonderful to be able to offer a new treatment for this disabling condition,” said Dr. William O’Neill.
“This device represents a breakthrough clinical and academic achievement,” said Tiberio Frisoli, M.D., Medical Director of Structural Heart Disease at Henry Ford Health. “We are excited to treat more patients now and to learn more about this disease process.”
For more information: henryford.com/services/structural-heart
Related Content:
Edwards Evoque Transcatheter Tricuspid Valve Replacement System Receives CE Mark
Edwards Announces One-year Data on Transfemoral Transcatheter Tricuspid Valve Replacement
Six-Month Outcomes Positive for Transfemoral Tricuspid Valve in Tricuspid Regurgitation Patients

 April 27, 2023
April 27, 2023