May 8, 2024 — 4C Medical Technologies, Inc. ("4C Medical"), a medical device company dedicated to advancing minimally ...
Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
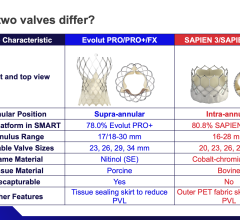
May 7, 2024 — Medtronic announced the release of important clinical outcomes in two leading transcatheter valve ...
May 6, 2024 — Teleflex Incorporate, a leading global provider of medical technologies, today announced that the Wattson ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...

Clinical trials and innovative technology took center stage during the month of April, racking up some record number ...
April 25, 2024 —Heart-Valve-Surgery.com, a leading patient advocacy group for heart valve disease, with support from Med ...
April 25, 2024 — Atlantic Health System’s Morristown Medical Center treated the first patient in New Jersey using Edward ...
April 23, 2024 — Medtronic plc, a global leader in healthcare technology, today announced the launch of its latest ...
April 17, 2024 —CPR Therapeutics, Inc. (CPR-T), an early-stage medtech startup funded by the N.I.H and N.S.F to develop ...
April 16, 2024 — Vivasure Medical, a company pioneering novel fully absorbable technology for percutaneous vessel ...
April 9, 2024 — UC Davis Health cardiology team members are among the first in the country to treat patients with tricus ...
April 9, 2024 — Administering tranexamic acid (TxA), a drug used to reduce bleeding during heart surgery, topically ...
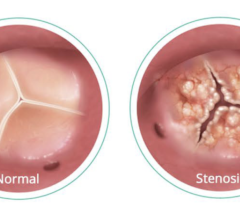
April 9, 2024 — People with a small aortic annulus, a part of the heart’s anatomy where the left ventricle meets the ...
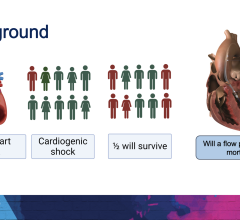
April 8, 2024 — Implantation of the Impella CP micro-axial flow pump in the hours after a heart attack significantly ...
April 4, 2024 — Osso VR, a leader in immersive procedural training, announces the release of a co-developed curriculum ...

Here is a Top 10 look at the content that was trending during the month of March on dicardiology.com:
1. CLS Health ...


 May 08, 2024
May 08, 2024