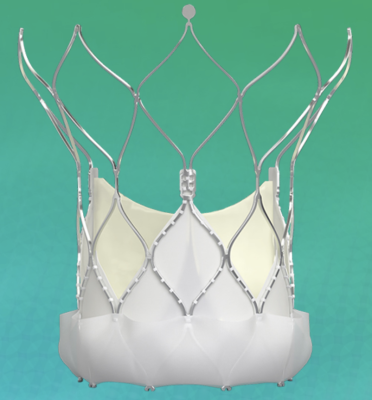
March 25, 2024 — Abbott announced the first patient has been enrolled in the ENVISION investigational device exemption (IDE) clinical trial. The global, randomized trial (envisiontrial.com) will evaluate the safety and effectiveness of Abbott’s minimally invasive Navitor transcatheter aortic valve implantation (TAVI) system in approximately 1,500 patients at intermediate or low surgical risk with severe aortic stenosis (narrowing of the aortic valve). Currently, 2 out of 3 of people undergoing TAVI in the U.S. fall into this category.
The trial will be used to support expanded indication for the Navitor TAVI system’s treatment of aortic stenosis across surgical risk categories. The Navitor Vision valve recently launched in the U.S. and features radiopaque markers that help physicians with implanting the device.
Aortic stenosis is one of the most common and life-threatening heart valve diseases. As the world’s population continues to age, cases of aortic stenosis are projected to double in the U.S. in the next few decades, underscoring the need for expanded treatment options.
For more information: www.abbott.com


 July 08, 2024
July 08, 2024 









