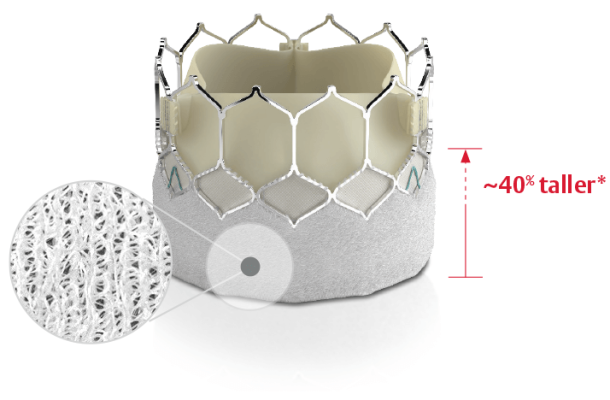
September 26, 2022 — Edwards Lifesciences announced the launch of the SAPIEN 3 Ultra RESILIA valve, which incorporates Edwards' breakthrough RESILIA tissue technology with the industry-leading SAPIEN 3 Ultra transcatheter aortic heart valve. The launch follows recent U.S. Food and Drug Administration(FDA) approval.
RESILIA tissue is a bovine pericardial tissue treated with advanced anti-calcification technology and serves as the platform for Edwards' new class of valves, including the next generation SAPIEN X4 valve, which is currently undergoing clinical trials. RESILIA tissue delivers important advances to the SAPIEN 3 Ultra platform including enhanced calcium blocking properties and dry tissue packaging conditions that facilitate ease of use. RESILIA tissue has demonstrated freedom from structural valve deterioration at 5 years and provides the potential to extend the durability of the SAPIEN 3 Ultra RESILIA valve. It is used in the world's leading surgical aortic valve, the Edwards INSPIRIS RESILIA valve.
"The SAPIEN 3 Ultra RESILIA valve builds on Edwards' 40 years of leadership in tissue technology by combining advancements in tissue science with the industry leading SAPIEN 3 Ultra valve to offer the only dry storage transcatheter heart valve on the U.S. market today," said Larry Wood, corporate vice president, transcatheter aortic valve replacement. "The RESILIA tissue's anti-calcification technology addresses one of the primary causes of reintervention following heart valve replacement. The SAPIEN 3 Ultra RESILIA valve is a prime example of Edwards' continued focus on innovating to meet the current and future needs of patients to help them live longer, healthier and more productive lives."
The SAPIEN 3 Ultra RESILIA valve will be available in the U.S. in limited release in the fourth quarter of 2022. The commercial opportunity related to this approval is factored into 2022 financial expectations.
For more information: www.edwards.com


 October 31, 2025
October 31, 2025 









