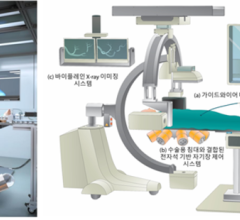
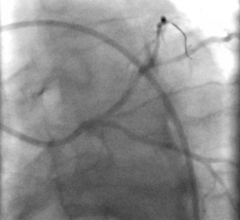
The global interventional cardiology device market is driven by the number of procedures performed across the globe. The market is primarily driven by the number of angiography and angioplasty ...
Guidewires
This channel includes news and new technology innovations for guide wires used in interventional cardiology and interventional radiology. Guidewires are used to navigate vessels to target lesions so larger, less maneuverable treatment devices can be pushed over the wire to rapidly reach the lesion.
Dec. 3, 2025 — Atraverse Medical, a medical device company developing next-generation left-heart access technology, has ...
June 17, 2024 — Medtronic launched the Steerant Aortic Guidewire, tailored to facilitate catheter placement and exchange ...
May 6, 2024 — Teleflex Incorporate, a leading global provider of medical technologies, today announced that the Wattson ...
The global interventional cardiology device market is driven by the number of procedures performed across the globe. The ...
December 23, 2022 — Electroducer, a company developing an innovative and cost-effective medical device for the treatment ...
June 17, 2022 — A multidisciplinary team of robotics and electronic systems engineers working with cardiologists and ...
April 12, 2022 — Transit Scientific announced the FDA clearance of its XO Cross Support Catheter Platform to include ...
September 20, 2021 — The American Heart Association (AHA) announced Sept. 16 it decided to convert from a planned in ...
August 18, 2020 — Philips Healthcare introduced its new OmniWire, the world’s first solid core fractional flow reserve ...
February 19, 2020 — Connectiv, a division of The Software and Information Industry Association (SIIA), has announced the ...
October 21, 2019 — Cardiovascular Systems Inc. recently announced U.S. Food and Drug Administration (FDA) premarket ...
October 11, 2019 — The U.S. Food and Drug Administration (FDA) issued two final guidances for the performance testing ...

September 3, 2019 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...

Guidewire engineering has become more advanced over the past decade as interventional cardiologists have advanced their ...
May 21, 2019 – Medtronic plc announced its entrance into the guide extension catheter market with the global launch of ...




 December 05, 2025
December 05, 2025