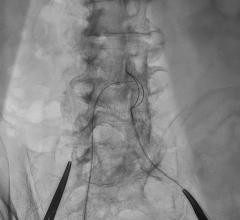
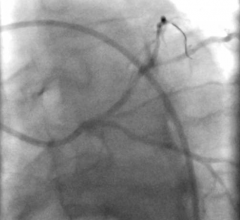
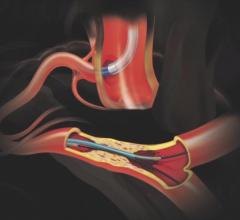
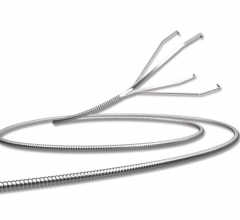
May 21, 2019 – Medtronic plc announced its entrance into the guide extension catheter market with the global launch of ...
Guidewires
This channel includes news and new technology innovations for guide wires used in interventional cardiology and interventional radiology. Guidewires are used to navigate vessels to target lesions so larger, less maneuverable treatment devices can be pushed over the wire to rapidly reach the lesion.
April 18, 2019 — The U.S. Food and Drug Administration published a draft guidance titled, “Technical Considerations for ...
April 10, 2019 — According to a new research study, straight tip guidewires, with a current share of more than a third ...
In 2009, the GuideLiner Catheter revolutionized the concept of guide extension, creating new possibilities in ...
June 14, 2018 — The U.S. Food and Drug Administration (FDA) released the following two draft guidance documents:
- Coro ...
January 24, 2018 — Cardiovascular Systems Inc. recently announced two new partnerships broadening the company’s product ...
October 5, 2017 — BTG plc announced it has acquired Roxwood Medical, provider of advanced cardiovascular specialty ...
June 22, 2017 — The U.S. Food and Drug Administration (FDA) has identified Vascular Solutions’ recent recall of its ...
May 30, 2017 — A Canadian cardiologist has published a report in the journal Eurointervention describing how he used a ...
March 20, 2017 — Teleflex Inc. recently announced 510(k) clearance by the U.S. Food and Drug Administration (FDA) and U ...
December 7, 2016 — Teleflex Inc. and Vascular Solutions Inc. announced that the companies have entered into a definitive ...
A discussion with guidewire expert Dimitri Karmpaliotis, M.D., Ph.D., FACC, about the basics of interventional guidewire ...
November 9, 2016 — Merit Medical Systems Inc. announced that it has received 510(k) clearance for the SwiftNINJA ...
October 18, 2016 — Medtronic plc announced last week that it has notified customers of a voluntary recall of certain ...
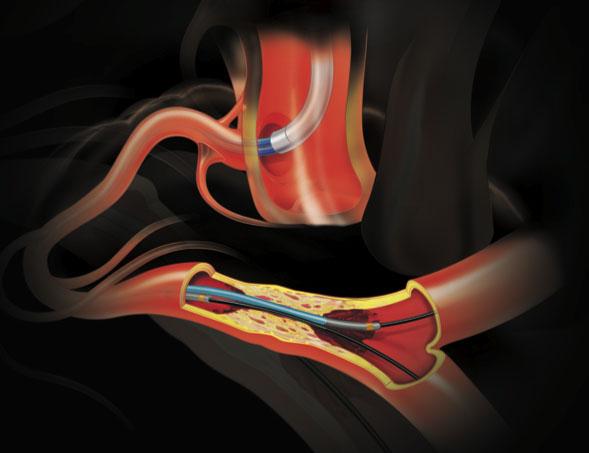
While guidewires are a key tool used by all interventionalists in the cath lab, most operators do not have a deep ...


 May 21, 2019
May 21, 2019