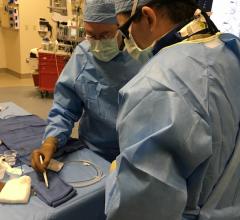
December 23, 2022 — Electroducer, a company developing an innovative and cost-effective medical device for the treatment of heart diseases, today announces that the positive results from its pilot study have been published in the medical journal EuroIntervention.
EuroIntervention is well regarded globally as the most prestigious European publication focused on interventional cardiology.
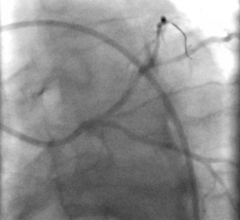
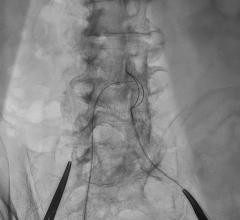
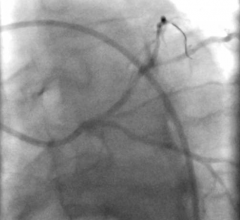
Titled ‘A direct wire pacing device for transcatheter heart valve and coronary interventions: a first-in-human, multicentre study of the Electroducer Sleeve’, the study involved 60 patients from four French medical centers (the Clinique Pasteur in Toulouse, the Cardiovascular Institute in Grenoble, the Médipôle Lyon-Villeurbanne Hospital in Villeurbanne and the Jacques Cartier private Hospital in Massy). It was the first of its kind in the world and confirmed that the Electroducer Sleeve is a safe and effective treatment for heart valve diseases and complex coronary interventions.
The Electroducer Sleeve makes it simpler and safer to perform endovascular procedures requiring temporary cardiac pacing. Implanting a temporary pacemaker is the current conventional technique. This is an invasive procedure that carries additional risks and increases procedural time and radiation exposure for the patient. In the future, the Electroducer Sleeve could replace this step by enabling the heart to be stimulated directly through the ‘guidewire’, which is used to deliver the valve or stent. This combined approach simplifies the procedure and reduces the risk of complications at the same time. According to a study published in 20191, it also reduces the total cost of the procedure by around 12%.
Dr. Jérôme Wintzer-Wekehind, principal investigator on the study and interventional cardiologist at the Cardiovascular Institute in Grenoble, said: “As well as offering benefits for patients, this device is simple and universal — these advantages mean there will certainly be widespread uptake and usage.”
Dr. Nicolas Dumonteil, one of the investigators on the study and interventional cardiologist at the Clinique Pasteur in Toulouse, added: “I am confident that in a few years’ time, Electroducer Sleeve will replace our conventional technique, providing physicians and patients with a faster, safer and simpler procedure.”
Dr. Benjamin Faurie, CEO and founder of Electroducer, said: “Having our study results published in such a prestigious medical journal shows that our clinical approach has tremendous potential to become the procedure of choice. It is a great opportunity to present the benefits of our solution to the global cardiology community. We plan to bring the Electroducer Sleeve to market within the next year, so that as many patients and cardiologists as possible can take advantage of our technology.”
The Electroducer Sleeve is expected to reach the US market in 2023, followed by the European market in 2024, once it receives FDA authorization and a CE marking respectively. The device has the potential to be used in nearly 1.5 million interventions per year worldwide by 2025. The company aims to capture 8% of this market, representing around 120,000 procedures per year, by this date.For more information: www.electroducer.io
Reference:
1 Benjamin Faurie, Géraud Souteyrand, Patrick Staat, Matthieu Godin, Christophe Caussin, Eric Van Belle, Lionel Mangin, Pierre Meyer, Nicolas Dumonteil, Mohamed Abdellaoui, Jacques Monségu, Isabelle Durand-Zaleski, Thierry Lefèvre and for the EASY TAVI Investigators. Left Ventricular Rapid Pacing Via the Valve Delivery Guidewire in Transcatheter Aortic Valve Replacement, Journal of American College of Cardiology (JACC), Volume 12, Issue 24 (2449-2459), December 2019.


 June 17, 2024
June 17, 2024