Technology-enabled “Insourcing” approach to cardiac arrhythmia diagnosis provides patient and provider benefits
The incidence and prevalence of atrial fibrillation (AF) have increased over the past 20 years. Studies suggest it will continue to do so in the future, adding significantly to the global burden of cardiac disease.1 Earliest detection and treatment of AF can reduce the risk of disabling stroke, heart failure and sudden cardiac death.
In the U.S., typically when a provider prescribes a multi-day ambulatory ECG patch or event monitor to check for the presence of AF or other cardiac arrhythmias, staff apply the device to the patient in the clinic. The patient then returns the monitor after wearing it for a predetermined time. Device interrogation and draft reporting of ECG data is commonly outsourced to a third-party ECG service center company (‘IDTF’) that processes results and bills payers for the large technical component of the ambulatory ECG procedure code.
Due to staffing shortages and other logistics challenges at IDTFs, results reporting can take many days, weeks, or even more than a month before physicians receive patient results. Diagnostic delays, patient data breaches and fragmented care are often the undesired effects of outsourcing ECG services.
Thanks to recent innovations in automated ECG analysis algorithm technology, providers can now bring long-term ECG patch data analysis and draft reporting entirely in-house, streamlining the process and reducing overall time to patient diagnosis. ‘Insourcing’ rather than outsourcing ECG services can also reduce operational costs and maximize reimbursement for providers.
“One of the main reasons ECG data analysis had been outsourced was because the technology previously didn’t exist to enable efficient automated processing of large amounts of ECG data in the clinical setting. A typical 7-day continuous ECG recording can exceed over a million beats per test,” said Val Kapitula, a Cardiovascular Systems expert at Paragon Consulting Partners. “Point-of-care ECG analysis capabilities just weren’t there, and the market wasn't ready when the first prescription ECG patches were launched. IDTFs were needed then, but not necessarily now, given today’s technology and ease of use.”
“Now you can prescribe an ECG patch and reach a diagnosis in just over a week, with all ECG data local and accessible right in your office or healthcare system,” Kapitula said. “Patients can wear an ECG patch for 7 days, return the device to their local healthcare provider for processing, with results available for physician review and interpretation within minutes. There’s virtually no waiting, which is more efficient for the physician practice and staff. From a patient perspective in terms of getting to a diagnosis, it’s beautiful.”
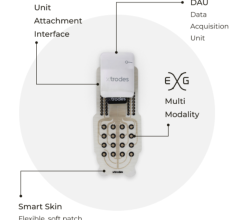
The Cardea SOLO ECG System from Cardiac Insight combines the only single-use wearable ECG Sensor specifically designed for use with in-office automated Cardea SOLO Analysis Software. The provider applies the SOLO Sensor to the patient and schedules a follow up visit in seven days. At the follow up appointment, staff remove the Sensor and connect it to a docking station that uploads all ECG data to the office-based Windows PC. The algorithm-based ECG software analyzes and automatically generates a draft summary report for provider review and interpretation in as little as five minutes, facilitating direct clinical decision-making in a single follow up visit.
Not only does this reduce the time to diagnosis and improve patient satisfaction, but Cardea SOLO provides a provider-centric care model that can recapture practice revenue streams that were previously lost to outsourcing “With this ‘insourcing’ approach, as they call it, providers have total control of the cardiac arrhythmia diagnostic process and can bill payers using the global payment code. This simplifies the practice’s billing as well as reimbursement payment processing by the payers.” Kapitula said.
Kapitula cautions that when evaluating an insource versus an outsource approach, a provider should consider their practice profile and fit for point-of-care solutions like Cardea SOLO ECG – whether it be staff accountability and education needs, software training, PC availability, informatics security, and data storage requirements.
“If the office is part of a larger IDN, it’s probably not a big lift, because they can most-likely consolidate the solution into their existing infrastructure. Smaller practices just need to consider all these implementation aspects. They typically have the advantage of speed and greater flexibility in adapting their care workflows, however.”
Ultimately, adding Cardea SOLO ECG test insourcing can deliver measurable clinical and economic benefits, as well as an improved patient experience to interested providers. “It’s an overall win-win and a great practice-building approach, particularly with patients who experience anxiety while waiting for critical test results. Patients and their physicians deserve timely testing that leads to diagnosis and earliest treatment. Insourcing ECG diagnostic procedures can address that need,” Kapitula said.
Editor's note: This blog is part of a series on advancements in wearable ECG technology. Click here to view additional articles in this series.
Reference:
1. Lippi, Giuseppe et al. “Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge.” International journal of stroke : official journal of the International Stroke Society vol. 16,2 (2021): 217-221. doi:10.1177/1747493019897870


 January 14, 2026
January 14, 2026