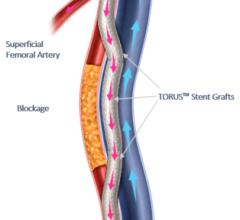
February 12, 2024 — Sensome, a company pioneering the connected medical device revolution with the world’s smallest ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
January 18, 2024 — Summa Therapeutics, LLC announced that the first-in-man injectable angioplasty procedures for ...
January 9, 2024 — Proceedings from an expert consensus roundtable that discussed the benefits of intravascular ...
November 10, 2023 —Getinge announced commercial availability of the iCast covered stent system in the United States for ...
November 9, 2023 — The VIVA Foundation, a not-for-profit organization dedicated to advancing the field of vascular ...
November 2, 2023 — The second round of late-breaking clinical trials results from the Vascular InterVentional Advances ...
October 24, 2023 — Four years ago, following a publication based on the limited data available, the U.S. Food and Drug ...
September 14, 2023 — LimFlow SA, a pioneer in the development of minimally-invasive technology for the treatment of chro ...
July 11, 2023 — The U.S. Food and Drug Administration (FDA) is informing healthcare providers about updated information ...
June 21, 2023 — Royal Philips, a global leader in health technology, announced it has teamed up with BIOTRONIK (Lake ...
June 21, 2023 — A new science report issued by the American Heart Association (AHA) emphasizes the importance of ...
June 16, 2023 — Endologix LLC, a privately held, global medical device company, dedicated to providing disruptive ...
June 15, 2023 — Endologix LLC, a privately held, global medical device company dedicated to providing disruptive ...
May 30, 2023 — The US FDA, on the 24th of May 2023, granted an Investigational Device Exemption (IDE) approval for Conce ...
May 19, 2023 — Findings from a study examining the relationship between marijuana use and peripheral artery disease (PAD ...

 February 12, 2024
February 12, 2024