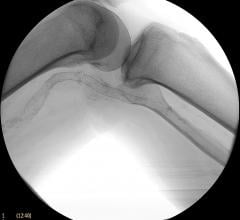
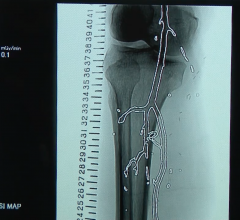
August 28, 2019 — The U.S. Food and Drug Administration (FDA) released an updated MedWatch Alert this month on the ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
August 27, 2019 — Concept Medical was recently granted Breakthrough Therapy designation by the U.S. Food and Drug ...
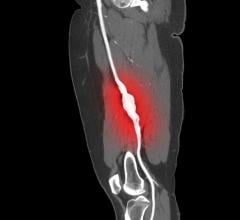
August 23, 2019 – Patients with peripheral artery disease (PAD) were significantly less likely to face amputation after ...
August 23, 2019 — Cook Medical recently released the second generation of the 2.6 Fr CXI Support Catheter with platinum ...
August 14, 2019 — Concept Medical Inc. (CMI) has been granted "Breakthrough Device Designation" from the U.S. Food and ...
August 13, 2019 — A simple change in the way health professionals track their patients’ progress has brought improved ...
August 13, 2019 — Non-invasive techniques and devices for assessing blood flow and other diagnostic considerations for ...
August 6, 2019 — Cardiovascular Systems Inc. (CSI) has acquired the Wirion Embolic Protection System and related assets ...
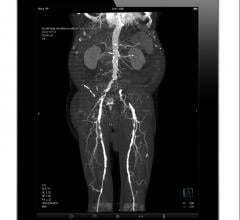
August 1, 2019 — Less-invasive procedures to open severely clogged leg arteries were as good at helping people survive ...
July 17, 2019 — A new analysis published by The Sage Group LLC concludes that the all-cause cost of critical limb ...
June 20, 2019 – VEITHsymposium and the Cardiovascular Research Foundation (CRF) announced an alliance between Transcathe ...
May 29, 2019 — Philips announced the three-year results from the ILLUMENATE Pivotal trial and the ILLUMENATE European ...
April 15, 2019 – Intact Vascular Inc. received U.S. Food and Drug Administration (FDA) market clearance for the Tack ...
February 27, 2019 — Ra Medical Systems Inc. announced a 98 percent success rate in the results from a 52-patient study u ...

The Society for Cardiovascular Angiography and Interventions (SCAI) 2019 Scientific Sessions in Las Vegas, May 19-22 ...


 August 28, 2019
August 28, 2019