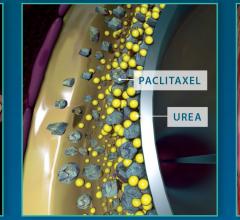
May 3, 2018 — The U.S. Food and Drug Administration (FDA) has expanded the indication for the Medtronic In.Pact Admiral ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
April 27, 2018 — Intact Vascular Inc. recently closed a Series C financing totaling $20 million. This financing is ...
March 23, 2018 — Patients with foot ulcers or gangrene who received the experimental drug JVS-100 did not show evidence ...
March 14, 2018 — A late-breaking analysis of the landmark COMPASS study presented at the American College of Cardiology ...
February 20, 2018 — Corindus Vascular Robotics Inc. announced today that it received 510(k) clearance from the U.S. Food ...
February 19, 2018 — A late-breaking analysis of the landmark COMPASS study was presented at the 2018 International ...
February 7, 2018 — Medtronic plc recently added to its body of clinical evidence supporting the In.Pact Admiral drug ...
February 7, 2018 — Teleflex Inc. has announced 510(k) clearance by the U.S. Food and Drug Administration (FDA) and U.S ...

February 1, 2018 – Thomas Zeller (Bad Krozingen, Germany) presented the 12-month results from Veryan Medical’s MIMICS-2 ...
February 1, 2018 – LimFlow SA, developer of minimally-invasive technology for the treatment of end-stage critical limb ...
January 26, 2018 – Medtronic plc announced the initiation of its investigational device exemption (IDE) study for the ...
January 24, 2018 — Cardiovascular Systems Inc. recently announced two new partnerships broadening the company’s product ...
January 9, 2018 – Avinger Inc. announced that Arne Schwindt, M.D., a vascular surgeon at St. Franziskus Hospital in ...
December 29, 2017 — iVascular announced the release of the new Oceanus 14 Pro percutaneous transluminal angioplasty (PTA ...

 May 03, 2018
May 03, 2018