April 27, 2018 — Intact Vascular Inc. recently closed a Series C financing totaling $20 million. This financing is designed to fund the company through pre-market approval (PMA) of the Tack Endovascular System for the treatment of post-angioplasty dissections above the knee.
Major participants in the Series C financing included New Enterprise Associates (NEA), H.I.G. BioVentures and Quaker Partners. The financing is structured in two stages or tranches, with the second tranche closing later in 2018.
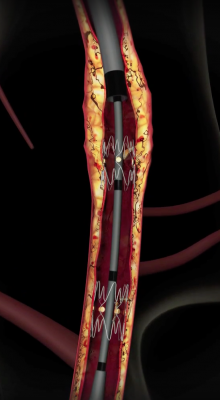
The Tack Endovascular System is a new solution for precision dissection repair following balloon angioplasty with a first-of-its-kind implant designed to help maintain vessel integrity and enhance blood flow. This promotes healing, improves outcomes and preserves limbs. Unrepaired dissections — which are frequent following balloon angioplasty — increase the probability of acute arterial occlusion and may continue narrowing the artery, which leads to lower long-term patency rates. The Tack Endovascular System leaves a minimal amount of metal in the artery, reduces mechanical stress on the arterial wall and preserves future treatment options.
Intact Vascular is sponsoring three clinical trials to evaluate its Tack Endovascular System: TOBA II, TOBA III and TOBA II BTK. TOBA II is investigating the combination of the Tack device with both plain and Bard Lutonix drug-coated balloon (DCB) angioplasty in the arteries above the knee, and completed enrollment in March 2017. TOBA III is investigating the combination of the Tack system with the Medtronic In.Pact Admiral DCB in the arteries above the knee (inclusive of long lesions), and is nearing full enrollment. TOBA II BTK is investigating the combination of the Tack device with plain balloon angioplasty in the arteries below the knee and is actively enrolling patients.
For more information: www.intactvascular.com


 November 14, 2025
November 14, 2025 









