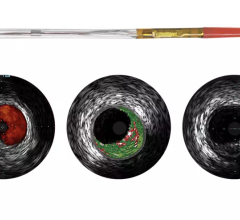
November 14, 2019 — The results of the 21 late-breaking clinical trials presented for the first time at Vascular Interventional Advances (VIVA) 2019 conference. These results were presented in four ...
Stents Peripheral
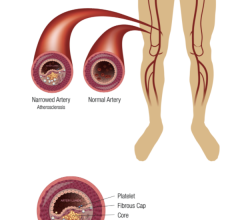
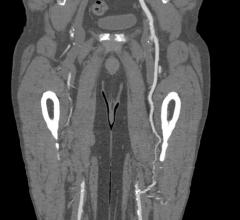
Peripheral stents are used to open narrow and hardening arteries that supply blood to the legs and feet.
May 30, 2024 — Biotronik announced the presentation of the 12-month results from the BIONETIC-I study this week at LINC ...
February 14, 2024 — Efemoral Medical, developer of advanced interventional bioresorbable therapies, today announced that ...
February 16, 2023 — Northeast Scientific Inc., the pioneers in reprocessing single use peripheral vascular catheters ...
October 11, 2022 — Efemoral Medical, developer of advanced interventional bioresorbable therapies, announced that it ...
August 23, 2022 — The first US patient has been enrolled in the FDA SELUTION4BTK (Below-the-Knee) clinical trial ...
February 22, 2021 — The U.S. Food and Drug Administration (FDA) recently cleared the Cook Medical Zilver Vena Venous ...
December 16, 2020 — Efemoral Medical announced the first-in-human (FIH) use of the its Efemoral bioresorbable vascular ...
September 4, 2020 — The U.S. Centers for Medicare and Medicaid Services (CMS) granted a New Technology Add-on Payment ...
June 16, 2020 — The 36-month results from Veryan Medical’s MIMICS-2 study for the BioMimics 3D femoropopliteal stent ...

Cardiovascular diseases (CVDs) are among the leading causes of death across the globe. For patients suffering from high ...
November 11, 2019 — Although common femoral artery (CFA) endarterectomy is still considered the gold standard treatment ...
November 7, 2019 — Although the Abbott Absorb fully bioresorbable drug-eluting stent (DES) was taken off the market due ...

November 6, 2019 – The final, long-term, patient-level data for the Cook Medical Zilver PTX drug-eluting stent (DES) ...
November 6, 2019 – Boston Scientific announced positive data for two of its devices within the peripheral drug-eluting ...





 May 30, 2024
May 30, 2024