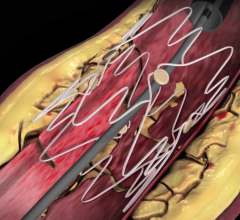
April 28, 2017 — Biotronik’s Pulsar-18 bare metal stent (BMS) has yielded high primary patency in a real-world setting ...
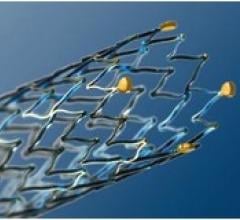
Stents Peripheral
Peripheral stents are used to open narrow and hardening arteries that supply blood to the legs and feet.
March 16, 2017 — W. L. Gore & Associates Inc. recently announced the Health Canada approval of the Gore Tigris Vascular ...
March 2, 2017 — Intact Vascular Inc. announced in February that its Tack Optimized Balloon Angioplasty II Below the Knee ...
December 13, 2016 — Biotronik announced the presentation of data confirming the efficacy of the Pulsar-18 bare metal ...
December 8, 2016 — LimFlow SA announced in November that it received the CE Mark for its fully percutaneous LimFlow ...

September 28, 2016 — The Cardiovascular Research Foundation (CRF) included 11 late-breaking trials and 16 first report ...

August 5, 2016 — Here are the top 20 most popular current content on the Diagnostic and Interventional Cardiology ...
August 2, 2016 —The U.S. Food and Drug Administration (FDA) granted market clearance for W. L. Gore & Associates’ Gore ...
June 7, 2016 — Intact Vascular Inc. announced that its Tack Optimized Balloon Angioplasty III (TOBA III) clinical trial ...
April 27, 2016 — Veniti Inc. announced the first successful treatment with the Vici Verto Venous Stent System of a ...
March 22, 2016 — PinnacleHealth CardioVascular Institute enrolled the first patient in Pennsylvania into the TOBA II ...
March 17, 2016 — Boston Scientific announced in late February that the Eluvia Drug-Eluting Vascular Stent System ...
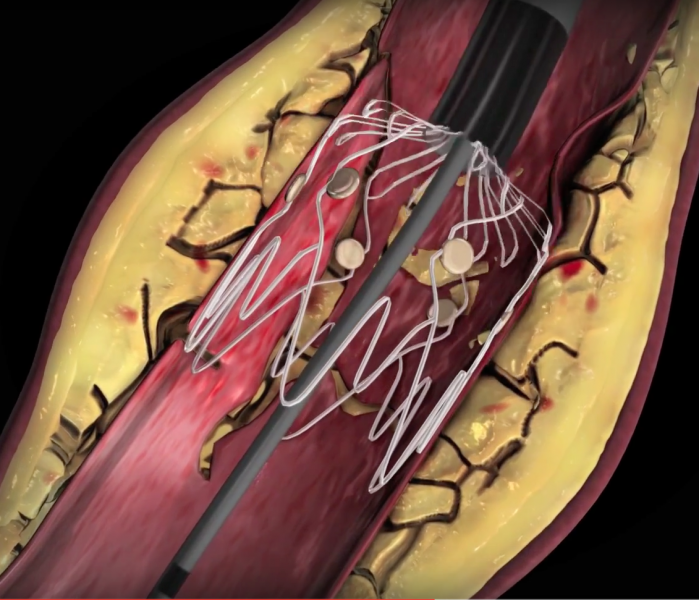
It is estimated that more than 10 million people in the United States are affected by peripheral arterial disease (PAD) ...
February 19, 2016 — Peripheral stents will account for over $4.6 billion in worldwide sales by 2020, according to a new ...
February 17, 2016 — Biotronik announced a partnership with Maquet Medical Systems USA to distribute Biotronik peripheral ...


 April 28, 2017
April 28, 2017