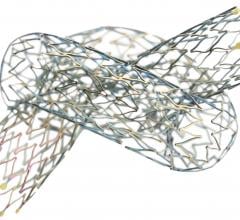
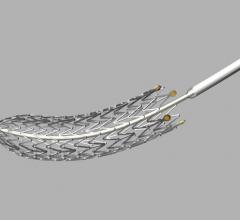
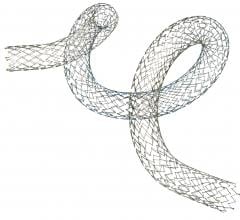
October 14, 2013 — Launching a clinical trial expected to serve as the foundation for global regulatory approvals, a ...
Stents Peripheral
Peripheral stents are used to open narrow and hardening arteries that supply blood to the legs and feet.
October 8, 2013 — Biotronik announced it has completed patient enrollment in the iliac arm of its Bioflex-I trial. Under ...
October 1, 2013 — The U.S. Food and Drug Administration (FDA) approved Medtronic Inc.'s Complete SE (self-expanding) ...

August 28, 2013 — Effective Oct. 1, Cook Medical’s Zilver PTX drug-eluting peripheral stent qualifies for new-technology ...
|
August 28, 2013 — According to Millennium Research Group (MRG), efforts to streamline the regulatory approval process in ... |
August 26, 2013 — Boston Scientific Corp. has completed enrollment in the SuperNOVA trial – a global, single arm ...
August 19, 2013 — Cook Medical is again shipping its Zilver PTX drug-eluting peripheral stent to medical centers in the ...
July 18, 2013 — Abbott announced that it has entered into an agreement to purchase IDEV Technologies, a privately held ...
March 21, 2013 — Cordis Corp. announced it has completed the acquisition of Flexible Stenting Solutions Inc. Currently ...
January 22, 2013 — Cordis Corporation announced the presentation of the two-year STROLL clinical study results at the ...

January 16, 2013 — Just weeks after the Food and Drug Administration (FDA) approved Cook Medical’s Zilver PTX drug ...
January 16, 2013 — Biotronik announced the first U.S. implant of their Pulsar-18 self expanding stent in the BIOFLEX-I ...
December 26, 2012 — Flexible Stenting Solutions Inc. (FSS) announced it gained CE mark in the European Union for its 6 ...
December 7, 2012 — More than half of patients treated for claudication or critical limb ischemia (CLI) with stenting of ...
November 20, 2012 — Cordis Corp. announced that the U.S. Food and Drug Administration (FDA) has approved the S.M.A.R.T ...


 October 14, 2013
October 14, 2013