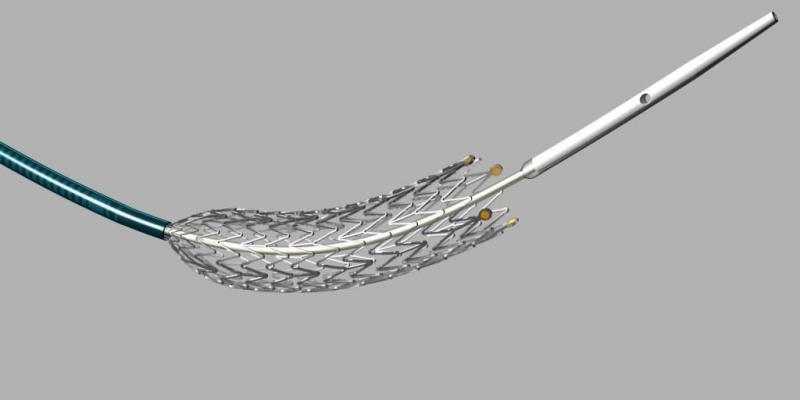
August 19, 2013 — Cook Medical is again shipping its Zilver PTX drug-eluting peripheral stent to medical centers in the United States, Japan, Europe and other major markets. The shipments follow a brief period of unavailability due to a voluntary recall by Cook related to an issue with the stent’s delivery catheter that has been resolved.
In 2009, Zilver PTX became the first drug-eluting peripheral stent in the world approved for peripheral artery disease (PAD) in the superficial femoral artery (SFA) when it was introduced to European physicians following CE mark approval. The device, which received a unanimous recommendation for approval from the circulatory device review panel of the U.S. Food and Drug Administration (FDA), was approved for U.S. sale in November 2012. In April 2012, it became the only drug-eluting peripheral stent approved for sale in Japan.
Tens of thousands of PAD patients in Europe, United States, Japan and more than 50 other markets around the world have been treated with the Zilver PTX stent. Cook’s device features a self-expanding stent of shape memory nitinol coated with the anti-cell proliferation drug paclitaxel. In more than three years of clinical study compared to bare metal stents, Zilver PTX has been shown to have a proven drug effect, reduced reintervention rates and longer-lasting vessel patency.
For more information: www.cookmedical.com


 July 02, 2024
July 02, 2024 









