November 15, 2012 — The U.S. Food and Drug Administration (FDA) granted market clearance for Cook Medical’s Zilver PTX ...
Stents Peripheral
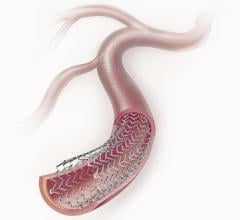
Peripheral stents are used to open narrow and hardening arteries that supply blood to the legs and feet.
October 23, 2012 — The use of stents for arterial blockages in the leg has been associated with high rates of late ...
October 15, 2012 — Three-year data from the Zilver PTX randomized controlled trial of paclitaxel-eluting stents for ...
October 8, 2012 — Following Health Canada approval, Cook Medical made the Zilver Vena Venous Self-Expanding Stent availa ...
September 27, 2012 — Boston Scientific has launched the Promus Element Plus BTK (Below The Knee) everolimus-eluting ...
August 22, 2012 — Placement of an endovascular stent has become a common procedure for successfully reopening blocked ...
August 15, 2012 — Previously unreleased three-year data from the Zilver PTX randomized controlled trial of paclitaxel ...
Aug. 8, 2012 — The U.S. Food and Drug Administration (FDA) has approved Abbott’s Omnilink Elite Vascular Balloon ...
July 12, 2012 — Terumo Interventional Systems, a strategic business unit of Terumo Medical Corporation, a U.S ...
July 3, 2012 — Flexible Stenting Solutions Inc., a leading developer of next generation peripheral arterial, venous ...
May 23, 2012 - Boston Scientific Corp. announces U.S. Food and Drug Administration approval and market launch of the ...
May 17, 2012 — Boston Scientific Corp. announces CE mark and European market launch of the Innova Self-Expanding Bare ...
March 16, 2012 — The U.S. Food and Drug Administration (FDA) approved the premarket approval (PMA) application for the ...
March 6, 2012 — In a development that brings advanced combination therapy treatment of peripheral artery disease (PAD) t ...
January 18, 2012 — Boston Scientific reported nine-month clinical endpoint data from its ORION trial, demonstrating ...

 November 15, 2012
November 15, 2012