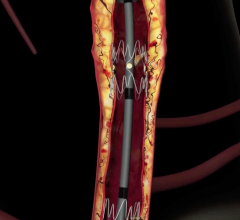
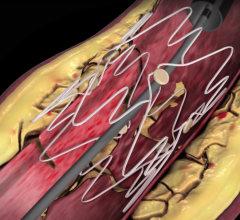
May 17, 2012 — Boston Scientific Corp. announces CE mark and European market launch of the Innova Self-Expanding Bare-Metal Stent System, which is designed to treat peripheral vascular lesions in arteries above the knee, specifically the superficial femoral artery (SFA) and proximal popliteal artery (PPA). The company plans to launch the product immediately in Europe and other CE mark countries.
"Treating arteries above the knee is difficult because the challenging anatomy can lead to stent fractures and higher restenosis rates," said Mauro Gargiulo, M.D., physician at Sant'Orsola-Malpighi in Bologna, Italy, who performed the first procedure using the Innova Stent in Europe. "The unique design and stent architecture used in the Innova Stent platform provide excellent radial strength, flexibility and durability which are critical to sustaining patency in treated SFA and PPA lesions. The excellent deliverability and placement accuracy add another significant level of benefit, especially when accessing challenging and long lesions."
The Innova Stent System consists of a nitinol, self-expanding, bare-metal stent loaded on an advanced low-profile delivery system. The innovative architecture features a closed-cell design at each end of the stent for more consistent deployment, and an open-cell design along the stent body for improved flexibility. Deployment accuracy is enhanced with a tri-axial catheter shaft designed to provide added support and placement accuracy as well as radiopaque markers to enhance visibility. The Innova Stent is 6 French compatible and is available in sizes from 5-8 mm in diameter and 20-200 mm in length.
"The Innova Stent is engineered to offer an advanced solution to treat blockages within these critical arteries," said Jeff Mirviss, president of Boston Scientific's peripheral interventions division. "This next-generation stent technology is designed to offer physicians improved acute performance and excellent long-term stent durability, intended to improve overall quality of life for patients with peripheral artery disease."
Patient enrollment continues in the SuperNOVA clinical trial to support the company's application for U.S. Food and Drug Administration approval of the Innova Stent System. This prospective, single-arm, non-randomized trial evaluates the safety and effectiveness of the Innova Stent in patients with stenosis of the SFA, PPA, or both. Enrollment is planned for up to 300 patients at 50 sites in the United States, Canada and Europe, and is expected to be completed in the first half of 2013.
Peripheral vascular disease (PVD) is a circulatory disorder that results from a build-up of plaque in one or more of the arteries of the legs. As the disease progresses, plaque accumulation may significantly reduce blood flow through the arteries, resulting in pain and increasing disability. In Europe, PVD affects 13 million people or one in 20 people over 40 years old.
In the United States, the Innova Stent System is an investigational device, limited by applicable law to investigational use only and not available for sale.
For more information: www.bostonscientific.com


 November 06, 2019
November 06, 2019