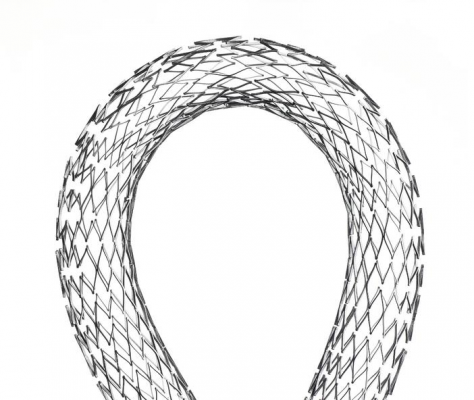
March 16, 2012 — The U.S. Food and Drug Administration (FDA) approved the premarket approval (PMA) application for the EverFlex self-expanding stent system from Covidien to treat peripheral artery disease (PAD). The device is intended to improve luminal diameter in the treatment of symptomatic de novo or restenotic lesions up to 180 mm in length in the native superficial femoral artery (SFA) and/or proximal popliteal arteries with reference vessel diameters ranging from 4.5 - 7.5 mm.
The EverFlex consists of a self-expanding nitinol stent premounted on an over-the-wire stent delivery system. The stent is a flexible self-expanding nitinol (nickel-titanium alloy) stent provided in multiple lengths and diameters.
The stent is pre-mounted on an 80 or 120 cm working length 6 French (.035-inch) over-the-wire (OTW) stent delivery system. Radiopaque markers on the stent delivery system aid in the accurate placement of the stent. Deployment is achieved by pulling the distal delivery system handle proximally, which retracts the outer sheath. The delivery system radiopaque stent retainer holds the stent stationary until the outer sheath is fully retracted to facilitate accurate placement. Upon deployment, the stent achieves its predetermined diameter and exerts a constant outward force to maintain patency in the target vessel.
The device is contra-indicated in patients with known hypersensitivity to nickel-titanium; patients in whom anticoagulant and/or antiplatelet therapy is contraindicated; and patients who are judged to have a lesion that prevents complete inflation of an angioplasty balloon or proper placement of the stent or stent delivery system.
The device was developed by ev3 prior to being acquired by Covidien.
For more information: www.ev3.net


 September 12, 2025
September 12, 2025 









