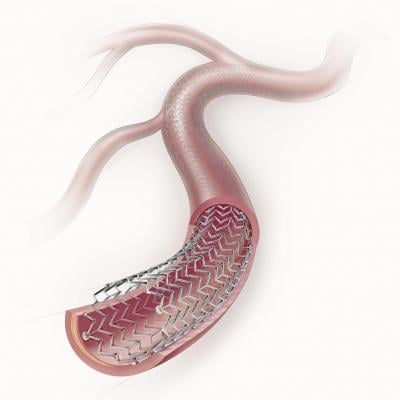
March 6, 2012 — In a development that brings advanced combination therapy treatment of peripheral artery disease (PAD) to Japanese patients for the first time, Cook Medical has received PMDA approval to sell the Zilver PTX Drug-Eluting Peripheral Stent in Japan. The device, indicated for treating PAD in the superficial femoral artery (SFA), is the first stent available in Japan approved for use in the SFA. Its approval also makes Zilver PTX the only drug-eluting peripheral stent available in that country.
“The Zilver PTX peripheral stent represents progress in treating PAD,” said Rob Lyles, vice president and global leader of Cook Medical’s Peripheral Intervention division. “Drug elution has come to the periphery for a reason. Clinical trials show that Zilver PTX has better long-term patency outcomes than bare-metal stents.”
Mitsuo Asami, vice president of Cook Japan and leader of Cook Japan’s Peripheral Intervention division added, “We are proud that this approval will bring Zilver PTX to Japanese physicians and patients. We intend to continue to support all medical practitioners by providing safe and minimally invasive treatment options for PAD.”
In a cooperative, multinational regulatory effort, a clinical trial for this product was carried out by Cook in Japan, the United States and Germany, with data from the trials being used to support regulatory submissions for Zilver PTX in Japan, the United States and Europe. The Zilver PTX Drug-Eluting Peripheral Stent offers physicians treating PAD in the SFA the combination of mechanical support via stenting with the drug paclitaxel to reduce the risk of restenosis. It is under U.S. Food and Drug Administration review and is not available for sale in the U.S. It received CE mark approval in August 2009. The device is now available in more than 45 countries.
For more information: www.cookmedical.com


 July 02, 2024
July 02, 2024 









