March 2, 2015 — Cardinal Health announced plans to acquire Johnson & Johnson’s Cordis business, a leading global ...
Stents Peripheral
Peripheral stents are used to open narrow and hardening arteries that supply blood to the legs and feet.
November 11, 2014 — Five-year results from the largest and longest-running clinical trial of a drug-eluting stent for ...

Nov. 11, 2014 — Viva Physicians, a not-for-profit organization in the field of vascular medicine and intervention ...
Oct. 27, 2014 — The U.S Food and Drug Administration (FDA) cleared the EverFlex Self-Expanding Peripheral Stent System ...
October 23, 2014 — The use of stents has improved management and outcomes of coronary artery disease, and clinical ...
September 9, 2014 — Cook Medical has enrolled the first patient in the clinical study in China of its Zilver PTX Drug ...
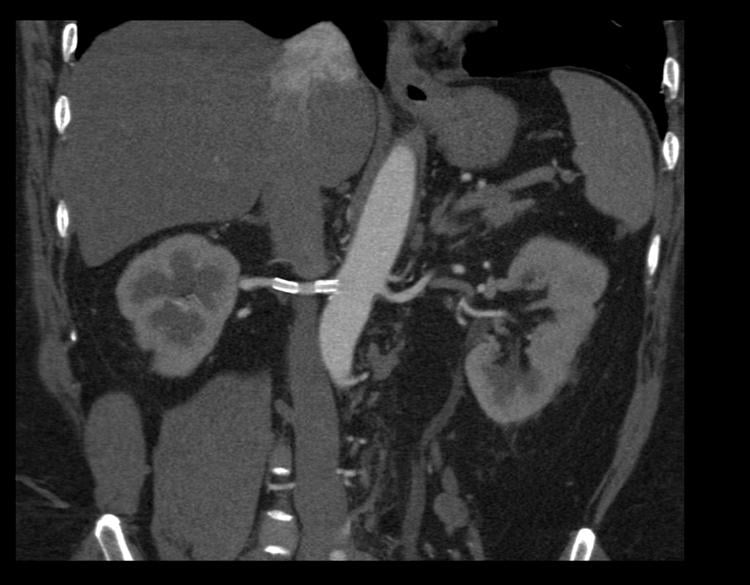
August 28, 2014 — Renal artery stenting to open blockages in the kidney arteries may benefit patients who have ...
August 8, 2014 — Biotronik announced the completion of patient enrollment in the superficial femoral artery (SFA) arm of ...
April 1, 2014 — The U.S. Food and Drug Administration (FDA) approved Abbott’s Supera Peripheral Stent System to treat ...
January 23, 2014 – Six-month results of the ESPRIT trial suggest a bioresorbable drug-eluting scaffold is effective in ...
October 23, 2013 — Vascular Interventional Advances (VIVA) Physicians, a not-for-profit organization dedicated to ...
October 21, 2013 — Bard Peripheral Vascular has sent an urgent Class I medical device recall notification letter ...


 March 02, 2015
March 02, 2015