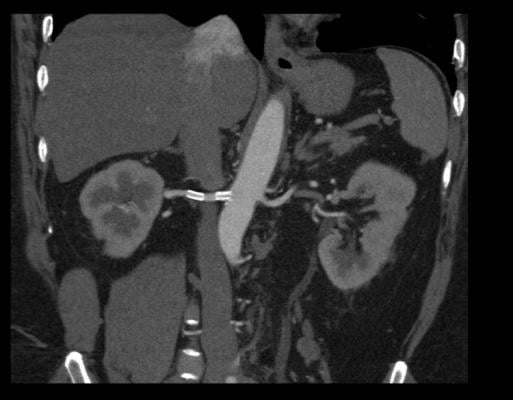
August 28, 2014 — Renal artery stenting to open blockages in the kidney arteries may benefit patients who have historically been excluded from modern clinical trials, according to new recommendations published online in Catheterization and Cardiovascular Interventions by the Society for Cardiovascular Angiography and Interventions (SCAI).
Blockages in the renal arteries are often asymptomatic, but may lead to high blood pressure or worsening of high blood pressure control. If left untreated, the disease can cause kidney failure and heart failure. Optimal medical therapy remains the preferred first-line treatment.
For patients whose condition is not controlled by medication, treating blocked arteries with angioplasty and stenting may help reduce blood pressure and prevent progression of the disease. Recent randomized controlled trials, including the Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial, have failed to demonstrate benefit of renal stenting over optimal medical therapy. However, there were patients whose condition might be improved by renal artery stenting who were excluded from the trial.
“The CORAL trial answered many of our questions about renal artery stenting, but some patients who are seeking treatment today were not included in CORAL, including patients in whom optimal medical therapy failed,” said Sahil A. Parikh, M.D., FSCAI, assistant professor of medicine, Case Western Reserve University School of Medicine, director, Interventional Cardiology Fellowship Program, University Hospitals Case Medical Center, and the paper’s lead author. “The new recommendations were developed to help physicians evaluate treatment options for the broad range of patients with renal artery disease.”
Based on an expert panel review of scientific data, the document recommends that patients most likely to benefit from renal artery stenting are those with cardiac disturbance syndrome or “flash” pulmonary edema (the rapid onset of fluid buildup in the lungs); patients whose high blood pressure has not been controlled by three or more medications at maximal tolerated doses; and those with blockages in both kidneys or severe blockages in a single functioning kidney where blood pressure or renal dysfunction cannot be managed medically.
In contrast, the expert panel recommends that patients with mild or moderate blockages (less than 70 percent), those with long-standing loss of blood flow and those with complete blockage of the renal artery are typically not good candidates for renal artery stenting. It is unknown whether renal artery stenting can improve symptoms in patients with heart failure over the long term.
In addition, the expert consensus statement reviews the evidence and expert opinion on the performance of renal artery angiography and intervention with stents. The panel delineates the best practices for assessing arterial narrowing that is intermediate (i.e. 50-70 percent), which often does not limit blood flow.
“Most practicing physicians performing these procedures will find this guidance particularly helpful in assessing renal artery disease in the future,” said Parikh.
The paper is the fourth in a series of recommendations developed by SCAI on treatment for peripheral artery disease (PAD). The incidence of PAD is growing, and the series aims to help interventional cardiologists determine the best course of treatment for patients whose blocked arteries place them at risk of reduced blood flow that can lead to loss of a limb or organ damage.
“As our understanding of PAD and its treatment options grows, physicians have the ability to improve symptoms and quality of life for many more patients today,” said SCAI 2014-15 President Charles Chambers, M.D., FSCAI. “Our series of treatment recommendations is designed to help interventional cardiologists provide the best possible care based on each patient’s individual symptoms and condition.
For more information: www.scai.org


 November 24, 2025
November 24, 2025 









